Abstract
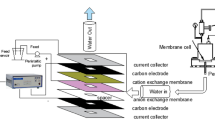
In the context of increasing heavy metal pollution (e.g., Cu2+, Cd2+, Pb2+ and Zn2+) in Liao River Basin of China in recent years due to metallurgical refinery, this study investigated the removal of Cu2+ from aqueous solution by Donnan dialysis process. Na+ was selected as the counterion, while GEFC-107 proton exchange membrane (GEFC Co., China) was used as the ion exchange membrane. Transport of Cu2+ from feed phase to receiver phase was enhanced by increasing \({{{\text{Na}}_{\text{R}}^{ + } } \mathord{\left/ {\vphantom {{{\text{Na}}_{\text{R}}^{ + } } {{\text{Cu}}_{\text{F}}^{{ 2 { + }}} }}} \right. \kern-0pt} {{\text{Cu}}_{\text{F}}^{{ 2 { + }}} }}\) ratio (subscripts R and F denote as receiver and feed phases, respectively). For the initial Cu2+ concentration of 200 mg L−1 and \({{{\text{Na}}_{\text{R}}^{ + } } \mathord{\left/ {\vphantom {{{\text{Na}}_{\text{R}}^{ + } } {{\text{Cu}}_{\text{F}}^{{ 2 { + }}} }}} \right. \kern-0pt} {{\text{Cu}}_{\text{F}}^{{ 2 { + }}} }}\) ratio of 20:1, a removal efficiency of 95.31 % was achieved for 190 min dialysis. The adsorption percentage by the proton exchange membrane increased from 12.12 to 85.15 % when the initial Cu2+ concentration decreased from 200 to 20 mg L−1. The kinetics data for Cu2+ removal was best interpreted by the first-order model. The removal efficiency decreased from 89.09 to 72.43 % when the proton exchange membrane was reutilized three times without membrane washing using acid. Higher initial pH of receiver phase or lower initial pH of feed phase facilitated the Cu2+ transport from feed to receiver phase. The feasibility of Donnan dialysis collaborated with precipitation to remove Cu2+ was found to be not suitable for Cu2+ removal with high concentration, i.e., initial concentration 200 mg L−1, since only a removal efficiency of Cu2+ of 54.08 % was achieved.






Similar content being viewed by others
References
Ali T (2007) Removal of fluoride from water using anion-exchange membrane under Donnan dialysis condition. J Hazard Mater 141:814–818
Carmelo S, Giuseppe A, Elisa L, Dongmei Z, Yangfei Y, Richard BA (2008) Heavy metal separation with polymer inclusion membranes. J Membrane Sci 323:444–451
Chhavi A, Chaudhury S, Pandey AK, Goswami A (2012) Kinetic aspects of Donnan dialysis through Nafion-117 membrane. J Membrane Sci 415–416:681–685
Fonseca EF, Neto JA, Silva CG (2013) Heavy metal accumulation in mangrove sediments surrounding a large waste reservoir of a local metallurgical plant, Sepetiba Bay, SE, Brazil. Environ Earth Sci 70(2):643–650
Hirofumi M (1996) Donnan dialysis with ion-exchange membranes. I. Diffusion coefficients using ions of different valence. Sep Sci Technol 31(15):2117–2129
Hirofumi M (1997) Diffusion coefficients of ions through ion-exchange membranes for Donnan dialysis using ions of the same valence. Chem Eng Sci 52:1087–1096
Hirofumi M (1998) Diffusion coefficients of ions through ion exchange membrane in Donnan dialysis using ions of different valence. J Membrane Sci 141:101–110
Jacek Wiśniewski, Agnieszka Różańska, Tomasz Winnicki (2005) Removal of troublesome anions from water by means of Donnan dialysis. Desalination 182:339–346
Jacek AW, Małgorzata K, Sylwia L (2011) Donnan dialysis and electrodialysis as viable options for removing bromates from natural water. Desalination 281:257–262
Li Q, Zhou JL, Chen B, Huang B, Zeng XD, Zhan JH, Pan XJ (2014) Toxic metal contamination and distribution in soils and plants of a typical metallurgical industrial area in southwest of China. Environ Earth Sci 72(6):2101–2109
Mohsen-Nia M, Montazeri P, Modarress H (2007) Removal of Cu2+ and Ni2+ from wastewater with a chelating agent and reverse osmosis processes. Desalination 217:276–281
Molinari R, Poerio T, Pietro Argurio (2006) Selective removal of Cu2+ versus Ni2+, Zn2+ and Mn2+ by using a new carrier in a supported liquid membrane. J Membrane Sci 280:470–477
Mustapha H, Françoise P, Jacqueline S, Claude G (1999) Fluoride removal from diluted solutions by Donnan dialysis with anion-exchange membranes. Desalination 122:53–62
Mustapha H, Françoise P, Jacqueline S, Claude G (2000) Fluoride removal from waters by Donnan dialysis. Sep Purif Technol 18:1–11
Ozlem A, Ali T, Yunus C, Mustafa E (2009) Removal of nitrate from the aqueous phase by Donnan dialysis. Desalination 239:276–282
Rajangam V, Rajangam P, Dharmalingam S (2011) Separation of heavy metals from water samples using anion exchange polymers by adsorption process. Desalination 267:267–276
Saji J, Presada Rao T, Ramamohan TR, Reddy MLP (1999) Studies on the liquid–liquid extraction of iron(III) and titanium(IV) with 3- phenyl-4-benzoyl-5-isoxazolone. Talanta 50:1065
Song JH, Yeon KH, Moon SH (2007) Effect of current density on ionic transport and water dissociation phenomena in a continuous electrodeionization (CEDI). J Membrane Sci 291:165–171
Tugba SK, Kir E, Ozkorucuklu SP, Karamızrak Esin (2010) Preparation and characterization of P2FAn/PVDF composite cation-exchange membranes for the removal of Cr(III) and Cu(II) by Donnan dialysis. React Funct Polym 70:900–907
WHO (2004) Guidelines for drinking water quality, Geneva
Zhou Y, Nie H, Christopher B, He Z, Zhu L (2009) Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with α-ketoglutaric acid. J Colloid Interf Sci. 330:29–37
Acknowledgments
This work was supported by National Science Foundation of China (NSFC Grant NO. 21277134 and NO. 51208179) and Plan for Scientific Innovation Talent of Henan University of Technology (NO.2014CXRC04).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wan, D., **ao, S., Cui, X. et al. Removal of Cu2+ from aqueous solution using proton exchange membrane by Donnan dialysis process. Environ Earth Sci 73, 4923–4929 (2015). https://doi.org/10.1007/s12665-015-4214-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-015-4214-0