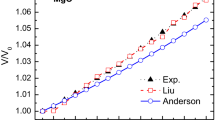
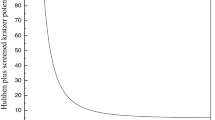
Abstract
A statistical-mechanical model for the entropy of charged hard-sphere mixture having arbitrary charges and sizes, based on a generalised bonding scheme, is applied to evaluate entropies and related thermal quantities of alkali halides, treating these compounds as large fractional charge-transferred ionic systems, where bonds in these hetero-diatomic molecules are modelled as a combination of strong partially charge-transferred ionic and feeble fractional covalent bonds. The computed theoretical results are in excellent agreement with the experimental data. This work has precisely incorporated the charging entropy contribution, opening an alternate approach for picturing bonds in hetero-diatomic univalent molecules.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article.
References
A Satpathy PhD Thesis Statistical Mechanics of Some Partially Ordered Systems (Jadavpur University, Kolkata, India) (1990)
N H March and M P Toshi Introduction to Liquid State Physics (Melville, NY: University Science Books) (2000)
D A McQuarrie Statistical Mechanics (University Science Books) (2000)
M Walz and D Spoel Physical Chemistry Chemical Physics 21 18516 (2019)
M Walz, M Ghahremanpour, P Maaren and D Spoel Journal of Chemical Theory And Computation 14 5933 (2018)
D Sirdeshmukh, L Sirdeshmukh and K Subhadra Alkali Halides: A Handbook of Physical Properties (Springer Science & Business Media) (2001)
G J Janz Molten Salts Handbook (Academic Press Inc.) (1967)
S J Yosim and B B Owens The Journal of Chemical Physics 41 2032 (1964)
G M Abernethy and M Silbert Physics And Chemistry of Liquids 11 195 (1982)
R V G Rao and A Satpathy Physica Status Solidi (b) 150 53 (1988)
A Satpathy Indian Journal of Physics 75 87 (2001)
A Satpathy and S Sengupta Indian Journal of Physics 89 323 (2014)
A Satpathy and S Sengupta Chemical Physics Letters 667 187 (2017)
L Pauling The Nature of the Chemical Bond and the Structure of Molecules and Crystals (Cornell University Press), Ch 3, Sec 3.1–3.7 pp 64–92 (1960)
L Pauling The Nature of the Chemical Bond and the Structure of Molecules and Crystals (Cornell University Press) Ch 3, Sec 3.9, pp 97–102 (1960)
L Pauling The Nature of the Chemical Bond and the Structure of Molecules and Crystals (Cornell University Press) Ch 1, Sec 1.3, pp 10–14 (1960)
A K Chandra Introductory Quantum Chemistry (Tata McGraw-Hill Publishing Co. Ltd., New Delhi, 4th ed.) Ch 7, Sec 7.7–7.18 pp 205–245 (1994)
L Pauling The Nature of the Chemical Bond and the Structure of Molecules and Crystals (Cornell University Press) Ch 6, Sec 6.5, pp 215–220 (1960)
L Pauling Proceedings of the Royal Society of London: Series A, Mathematical and Physical Sciences 356 433 (1977)
H Vermeeren Synthese 69 273 (1986)
A Satpathy J. Phys. Chem. Biophys. 7 (2017)
L Pauling The Nature of the Chemical Bond and the Structure of Molecules and Crystals (Cornell University Press) Ch 3, Sec 3.3, pp 74–78 (1960)
D Truhlar Journal of Chemical Education 84 781 (2007)
E G Visser, W van de Lugt and J T M de Hosson Journal of Physics F: Metal Physics 10 1681 (1980)
L Pauling The Nature of the Chemical Bond and the Structure of Molecules and Crystals (Cornell University Press), Ch 13, Sec 13.2–13.3, pp 511–532 (1960)
F G Fumi and M P Tosi Journal of Physics And Chemistry of Solids 25 31 (1964)
D Sirdeshmukh, L Sirdeshmukh and K Subhadra Alkali Halides: A Handbook of Physical Properties (Springer Science & Business Media) pp 7–9 (2001)
A Pratap, M Rani and N Saxena Pramana 30 239 (1988)
E Wilhelm Journal of Chemical Physics 60 3896 (1974)
G J Janz Molten Salts Handbook (Academic Press Inc.) pp 39–184 (1967)
D Sirdeshmukh, L Sirdeshmukh and K Subhadra Alkali Halides: A Handbook of Physical Properties (Springer Science & Business Media) pp 56–60 (2001)
G J Janz Molten Salts Handbook (Academic Press Inc.) p 356 (1967)
D Sirdeshmukh, L Sirdeshmukh and K Subhadra Alkali Halides: A Handbook of Physical Properties (Springer Science & Business Media) pp 136–138 (2001)
K Young and H Frederikse Journal of Physical And Chemical Reference Data 2 313 (1973)
D Sirdeshmukh, L Sirdeshmukh and K Subhadra Alkali Halides: A Handbook of Physical Properties (Springer Science & Business Media) p 74 (2001)
G J Janz Molten Salts Handbook (Academic Press Inc.) pp 1–30 (1967)
W Lugt Journal of Physics: Condensed Matter 8 6115 (1996)
J D Martin, S J Goettler, N Fossé and L Iton Nature 419 381 (2002)
Y Onodera, S Kohara, S Tahara, A Masuno, H Inoue, M Shiga, A Hirata, K Tsuchiya, Y Hiraoka, I Obayashi et al Journal of The Ceramic Society of Japan 127 853 (2019)
Acknowledgements
We are thankful to Mr. Uddipan Pahari, a graduate student in Mechanical Engineering at Maulana Abul Kalam Azad University of Technology, Kolkata, for hel** us to draw pictograms of the proposed bonding scheme using AutoCAD and one of the authors, Souradeep Satpathy is thankful to the National Institute of Science Education and Research (NISER), Bhubaneswar, India for providing some initial summer project contingency grant to start this work with Dr. Alok Satpathy.
Author information
Authors and Affiliations
Contributions
A.S.: Conceptualization, Methodology, Formal analysis, Validation, Writing, final draft editing, Supervision. S.S.: Data curation, Software, Computation, Visualization, Validation, Formal analysis, Writing—initial draft, editing and cross-checking.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satpathy, A., Satpathy, S. A model evaluation of entropies and related thermal quantities of alkali halides based on a generalised bonding scheme. Indian J Phys (2024). https://doi.org/10.1007/s12648-024-03142-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12648-024-03142-2