Abstract
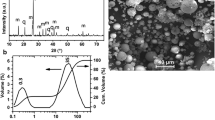
This study aims to inspect the effect of hydrothermal parameters on the zeolitization of coal fly ash and fumed silica using the L9 orthogonal Taguchi method. The Si/Al ratio, NaOH concentration, synthesis time, and hydrothermal temperature were considered as the operational parameters. The formed crystals were examined using XRD, FTIR, SEM–EDX, TG–DTA, BET, Raman microscopy, contact angle, and cation exchange capacity measurements. The effects of the different parameters were investigated by mean and variance analysis. The ideal levels of the zeolitization process were revealed to be high temperature (140 °C), medium concentration (1.5 M), lower Si/Al ratio (4), and long-time treatment (36 h). The zeolitization rate of started materials reached 84% and Na-P1 zeolite was the main neoformed phase with high crystallinity (104%). The obtained zeolite is found to be an effective and cheap adsorbent to deal with organic pollutants such as Methylene blue (33.74 mg/g) with a removal rate of 99.8%.
Graphical Abstract

Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included in the article.
References
Tironi A, Trezza MA, Irassar EF, Scian AN (2012) Thermal Treatment of Kaolin: Effect on the Pozzolanic Activity. Procedia Mater Sci 1:343–350. https://doi.org/10.1016/J.MSPRO.2012.06.046
Kaur B, Srivastava R, Satpati B (2015) Ultratrace detection of toxic heavy metal ions found in water bodies using hydroxyapatite supported nanocrystalline ZSM-5 modified electrodes †. R Soc Chem. https://doi.org/10.1039/c4nj02369b
Ji Y, Xu F, Wei W et al (2021) Efficient and fast adsorption of methylene blue dye onto a nanosheet MFI zeolite. J Solid State Chem 295:121917. https://doi.org/10.1016/J.JSSC.2020.121917
Marino G, Bergamini MF, Teixeira MFS, Cavalheiro ÉTG (2003) Evaluation of a carbon paste electrode modified with organofunctionalized amorphous silica in the cadmium determination in a differential pulse anodic strip** voltammetric procedure. Talanta 59:1021–1028. https://doi.org/10.1016/S0039-9140(03)00004-3
Rocha Junior CAF, Santos SCA, Souza CAG et al (2012) Synthesis of zeolites from boiler fly ash: Physical, chemical and mineralogical characterization. Ceramica 58:43–52. https://doi.org/10.1590/S0366-69132012000100008
Sathupunya M, Gulari E, Wongkasemjit S (2003) Na-A (LTA) zeolite synthesis directly from alumatrane and silatrane by sol-gel microwave techniques. J Eur Ceram Soc 23:1293–1303. https://doi.org/10.1016/S0955-2219(02)00287-X
Wu Y, Ren X, Wang J (2009) Facile synthesis and morphology control of zeolite MCM-22 via a two-step sol–gel route with tetraethyl orthosilicate as silica source. Mater Chem Phys 113:773–779. https://doi.org/10.1016/J.MATCHEMPHYS.2008.08.008
Abdullahi T, Harun Z, Othman MHD (2017) A review on sustainable synthesis of zeolite from kaolinite resources via hydrothermal process. Adv Powder Technol 28:1827–1840. https://doi.org/10.1016/J.APT.2017.04.028
Alkan M, Hopa Ç, Yilmaz Z, Güler H (2005) The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater 86:176–184. https://doi.org/10.1016/J.MICROMESO.2005.07.008
Rocha J, Klinowski J, Adams JM (1991) Synthesis of zeolite Na-A from metakaolinite revisited. J Chem Soc Faraday Trans 87:3091–3097. https://doi.org/10.1039/FT9918703091
Guisnet M, Pinard L (2018) Zeolites : From synthesis to applications. Chem React Eng 33:3–5. https://doi.org/10.51257/a-v2-j6675
Borhade AV, Kshirsagar TA (2017) Dholi AG (2017) Eco-Friendly Synthesis of Aluminosilicate Bromo Sodalite from Waste Coal Fly Ash for the Removal of Copper and Methylene Blue Dye. Arab J Sci Eng 4210(42):4479–4491. https://doi.org/10.1007/S13369-017-2759-9
Sun Z, Li C, Wu D (2010) Removal of methylene blue from aqueous solution by adsorption onto zeolite synthesized from coal fly ash and its thermal regeneration. J Chem Technol Biotechnol 85:845–850. https://doi.org/10.1002/JCTB.2377
Purnomo CW, Salim C, Hinode H (2012) Synthesis of pure Na–X and Na–A zeolite from bagasse fly ash. Microporous Mesoporous Mater 162:6–13. https://doi.org/10.1016/J.MICROMESO.2012.06.007
Mustakim SM, Das SK, Mishra J, et al (2020) Improvement in Fresh, Mechanical and Microstructural Properties of Fly Ash- Blast Furnace Slag Based Geopolymer Concrete By Addition of Nano and Micro Silica. Silicon https://doi.org/10.1007/s12633-020-00593-0
Jithendra C, Elavenil S (2020) Effects of Silica Fume on Workability and Compressive Strength Properties of Aluminosilicate Based Flowable Geopolymer Mortar under Ambient Curing. Silicon 12:1965–1974. https://doi.org/10.1007/s12633-019-00308-0
Calabrese L, Bonaccorsi L, Caprì A, Proverbio E (2014) Adhesion aspects of hydrophobic silane zeolite coatings for corrosion protection of aluminium substrate. Prog Org Coatings 77:1341–1350. https://doi.org/10.1016/J.PORGCOAT.2014.04.025
Sivalingam S, Kella T, Maharana M, Sen S (2019) Efficient sono-sorptive elimination of methylene blue by fly ash-derived nano-zeolite X: Process optimization, isotherm and kinetic studies. J Clean Prod 208:1241–1254. https://doi.org/10.1016/J.JCLEPRO.2018.10.200
Lin L, Lin Y, Li C et al (2016) Synthesis of zeolite/hydrous metal oxide composites from coal fly ash as efficient adsorbents for removal of methylene blue from water. Int J Miner Process 148:32–40. https://doi.org/10.1016/J.MINPRO.2016.01.010
Yao S, Zhang L, Zhu Y et al (2020) Evaluation of heavy metal element detection in municipal solid waste incineration fly ash based on LIBS sensor. Waste Manag 102:492–498. https://doi.org/10.1016/j.wasman.2019.11.010
Ahmaruzzaman M (2010) A review on the utilization of fly ash. Prog Energy Combust Sci 36:327–363. https://doi.org/10.1016/j.pecs.2009.11.003
Ferrarini SF, Cardoso AM, Paprocki A, Pires M (2016) Integrated Synthesis of Zeolites Using Coal Fly Ash: Element Distribution in the Products, Washing Waters and Effluent. J Braz Chem Soc 27:2034–2045. https://doi.org/10.5935/0103-5053.20160093
Król M, Mozgawa W, Morawska J, Pichór W (2014) Spectroscopic investigation of hydrothermally synthesized zeolites from expanded perlite. Microporous Mesoporous Mater 196, 216–222. https://doi.org/10.1016/j.micromeso.2014.05.017
Tabit K, Waqif M, Saâdi L (2019) Application of the Taguchi method to investigate the effects of experimental parameters in hydrothermal synthesis of Na-P1 zeolite from coal fly ash. Res Chem Intermed. https://doi.org/10.1007/S11164-019-03840-1
Musyoka NM, Petrik LF, Gitari WM et al (2012) Optimization of hydrothermal synthesis of pure phase zeolite Na-P1 from South African coal fly ashes. J Environ Sci Heal - Part A Toxic/Hazardous Subst Environ Eng 47:337–350. https://doi.org/10.1080/10934529.2012.645779
Irdhawati I, Suyanto H, Andani PY (2017) Zeolite-modified carbon paste electrode for determination of copper using anodic strip** voltammetry method. Alchemy J Penelit Kim 13:1. https://doi.org/10.20961/alchemy.v13i1.1808
Mintova S, Valtchev V (2002) Effect of the silica source on the formation of nanosized silicalite-1: an in situ dynamic light scattering study. Microporous Mesoporous Mater 55:171–179. https://doi.org/10.1016/S1387-1811(02)00401-8
Hosseini Asl SM, Javadian H, Khavarpour M et al (2019) Porous adsorbents derived from coal fly ash as cost-effective and environmentally-friendly sources of aluminosilicate for sequestration of aqueous and gaseous pollutants: A review. J Clean Prod 208:1131–1147. https://doi.org/10.1016/J.JCLEPRO.2018.10.186
Santasnachok C, Kurniawan W, Hinode H (2015) The use of synthesized zeolites from power plant rice husk ash obtained from Thailand as adsorbent for cadmium contamination removal from zinc mining. J Environ Chem Eng 3:2115–2126. https://doi.org/10.1016/j.jece.2015.07.016
Gooding OW (2004) Process optimization using combinatorial design principles: parallel synthesis and design of experiment methods. Curr Opin Chem Biol 8:297–304. https://doi.org/10.1016/J.CBPA.2004.04.009
Rida K, Bouraoui S, Hadnine S (2013) Adsorption of methylene blue from aqueous solution by kaolin and zeolite. Appl Clay Sci 83–84:99–105. https://doi.org/10.1016/J.CLAY.2013.08.015
Tan IAW, Hameed BH (2010) Adsorption isotherms, kinetics, thermodynamics and desorption studies of basic dye on activated carbon derived from oil palm empty fruit bunch. J Appl Sci 10:2565–2571. https://doi.org/10.3923/JAS.2010.2565.2571
Chen Q, Zhang Q, Yang Y et al (2021) Synergetic effect on methylene blue adsorption to biochar with gentian violet in dyeing and printing wastewater under competitive adsorption mechanism. Case Stud Therm Eng 26:101099. https://doi.org/10.1016/J.CSITE.2021.101099
Ghanadzadeh Gilani A, Ghorbanpour T, Salmanpour M (2013) Additive effect on the dimer formation of thiazine dyes. J Mol Liq 177:273–282. https://doi.org/10.1016/J.MOLLIQ.2012.09.005
Vara J, Ortiz CS (2016) Thiazine dyes: Evaluation of monomeric and aggregate forms. Spectrochim Acta Part A Mol Biomol Spectrosc 166:112–120. https://doi.org/10.1016/J.SAA.2016.05.005
Chang HL, Shih WH (2000) Synthesis of zeolites A and X from fly ashes and their ion-exchange behavior with cobalt ions. Ind Eng Chem Res 39:4185–4191. https://doi.org/10.1021/ie990860s
Satpati B, Kore R, Srivastava R (2014) ZSM-5 Zeolite Nanosheets with Improved Catalytic Activity Synthesized Using a New Class of Structure-Directing A. Chem Eur J 20:11511–11521. https://doi.org/10.1002/chem.201402665
Lin YW, Lee WH, Wang HH et al (2021) Environmentally friendly mesoporous material derived from thin-film transistor liquid crystal display and sandblasting and its application of environmental humidity control. J Am Chem Soc 1992;114:1. https://doi.org/10.21203/rs.3.rs-509521/v1
Ojha K, Pradhan NC, Samanta AN (2004) Zeolite from fly ash: Synthesis and characterization. Bull Mater Sci 27:555–564. https://doi.org/10.1007/BF02707285
Farahi A, Hammani H (2020) Electro-catalytic detection of dopamine at carbon paste electrode modified with activated carbon. Int J Environ Anal Chem 100:295–310. https://doi.org/10.1080/03067319.2019.1636043
Wahab MA, Guo W, Cho WJ, Ha CS (2003) Synthesis and characterization of novel amorphous hybrid silica materials. J Sol-Gel Sci Technol 27:333–341. https://doi.org/10.1023/A:1024077221572
Jan A, Pu Z, Khan KA et al (2022) A Review on the Effect of Silica to Alumina Ratio, Alkaline Solution to Binder Ratio, Calcium Oxide + Ferric Oxide, Molar Concentration of Sodium Hydroxide and Sodium Silicate to Sodium Hydroxide Ratio on the Compressive Strength of Geopolymer Concrete. Silicon 14:3147–3162. https://doi.org/10.1007/s12633-021-01130-3
Kazemian H, Naghdali Z, GhaffariKashani T, Farhadi F (2010) Conversion of high silicon fly ash to Na-P1 zeolite: Alkaline fusion followed by hydrothermal crystallization. Adv Powder Technol 21:279–283. https://doi.org/10.1016/j.apt.2009.12.005
González T, Ureta-Zañartu MS, Marco JF, Vidal G (2019) Effect of Zeolite-Fe on graphite anode in electroactive biofilm development for application in microbial fuel cells. Appl Surf Sci 467–468:851–859. https://doi.org/10.1016/j.apsusc.2018.10.120
Valášková M, Klika Z, Novosad B, Smetana B (2019) Crystallization and quantification of crystalline and non-crystalline phases in kaolin-based cordierites. Materials (Basel) 12:19–3104. https://doi.org/10.3390/ma12193104
Inada M, Eguchi Y, Enomoto N, Hojo J (2005) Synthesis of zeolite from coal fly ashes with different silica–alumina composition. Fuel 84:299–304. https://doi.org/10.1016/J.FUEL.2004.08.012
Lucovsky G (1987) Low-temperature growth of silicon dioxide films: A study of chemical bonding by ellipsometry and infrared spectroscopy. J Vac Sci Technol B Microelectron Nanom Struct 5:530. https://doi.org/10.1116/1.583944
Dodin M, Paillaud J-L, Lorgouilloux Y et al (2010) A Zeolitic Material with a Three-Dimensional Pore System Formed by Straight 12- and 10-Ring Channels Synthesized with an Imidazolium Derivative as Structure-Directing Agent. J Am Chem Soc 132:10221–10223. https://doi.org/10.1021/ja103648k
Kunecki P, Panek R, Wdowin M et al (2021) Influence of the fly ash fraction after grinding process on the hydrothermal synthesis efficiency of Na-A, Na-P1, Na-X and sodalite zeolite types. Int J Coal Sci Technol 8:291–311. https://doi.org/10.1007/s40789-020-00332-1
Li X, Han S, Xu J, Jiang N (2023) Green synthesis of nano-H-ZSM-5 zeolite single-crystal aggregates via an in situ reconstruction of the topology of natural clay. Microporous Mesoporous Mater 350:112441. https://doi.org/10.1016/J.MICROMESO.2023.112441
Al-Nahari S, Laurencin D, Alonso B (2023) Solvent-free synthesis of zeolites: New insights into the mechanism and non-mechanochemical route. Microporous Mesoporous Mater 350:112445. https://doi.org/10.1016/J.MICROMESO.2023.112445
Seghir S, Boulanger C, Diliberto S et al (2010) Enhancement of electrochemical transfer junction for cation extraction. Electrochem commun 12:1734–1737. https://doi.org/10.1016/J.ELECOM.2010.10.009
Liang X, Li Y, Yan W et al (2021) Preparation of SiC reticulated porous ceramics with high strength and increased efficient filtration via fly ash addition. J Eur Ceram Soc 41:2290–2296. https://doi.org/10.1016/j.jeurceramsoc.2020.11.039
Sellaoui L, Franco D, Ghalla H et al (2020) Insights of the adsorption mechanism of methylene blue on brazilian berries seeds: Experiments, phenomenological modelling and DFT calculations. Chem Eng J 394:125011. https://doi.org/10.1016/J.CEJ.2020.125011
Belaabed R, Elabed S, Addaou A et al (2016) Synthesis of LTA zeolite for bacterial adhesion. Boletín la Soc Española Cerámica y Vidr 55:152–158. https://doi.org/10.1016/J.BSECV.2016.05.001
Liu R, Dangwal S, Shaik I et al (2018) Hydrophilicity-controlled MFI-type zeolite-coated mesh for oil/water separation. Sep Purif Technol 195:163–169. https://doi.org/10.1016/J.SEPPUR.2017.11.064
Ninan N, Grohens Y, Elain A et al (2013) Synthesis and characterisation of gelatin/zeolite porous scaffold. Eur Polym J 49:2433–2445. https://doi.org/10.1016/J.EURPOLYMJ.2013.02.014
Goswami M, Phukan P (2017) Enhanced adsorption of cationic dyes using sulfonic acid modified activated carbon. J Environ Chem Eng 5:3508–3517. https://doi.org/10.1016/j.jece.2017.07.016
Khanal M, Rai D, Khanal R, Bhattarai A (2020) Determination of Point Zero Charge (PZC) of Homemade Charcoals of Shorea Robusta (Sakhuwa) and Pinus Roxburghii (Salla). Int J Eng Res Technol www.ijert.org 9 https://doi.org/10.1016/S1381-5148(99)00034-6
Laouini A, Jaafar-Maalej C, Limayem-Blouza I et al (2012) Preparation, Characterization and Applications of Liposomes: State of the Art. J Colloid Sci Biotechnol 1:147–168. https://doi.org/10.1166/jcsb.2012.1020
Syafalni S, Abustan I, Dahlan I et al (2012) Treatment of dye wastewater using granular activated carbon and zeolite filter. Modern Applied Sci 6:2–37. https://doi.org/10.5539/mas.v6n2p37
Huang C-H, Chang K-P, Ou H-D et al (2011) Adsorption of cationic dyes onto mesoporous silica. Microporous Mesoporous Mater 141:102–109. https://doi.org/10.1016/j.micromeso.2010.11.002
Yang YT, Tu CZ, Shi JY et al (2022) Cu(I)-organic framework as a platform for high-efficiency selective adsorption of methylene blue and reversible iodine uptake. J Solid State Chem 311:123133. https://doi.org/10.1016/J.JSSC.2022.123133
Khan MI (2020) Adsorption of methylene blue onto natural Saudi Red Clay: isotherms, kinetics and thermodynamic studies. Mater Res Express 7:55507. https://doi.org/10.1088/2053-1591/ab903c
Kumar A, Jena HM (2016) Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed-bed column. J Clean Prod 137:1246–1259. https://doi.org/10.1016/J.JCLEPRO.2016.07.177
Feng Y, Zhou H, Liu G et al (2012) Methylene blue adsorption onto swede rape straw (Brassica napus L.) modified by tartaric acid: Equilibrium, kinetic and adsorption mechanisms. Bioresour Technol 125:138–144. https://doi.org/10.1016/J.BIORTECH.2012.08.128
Chen H, Zhao J, Zhong A, ** Y (2011) Removal capacity and adsorption mechanism of heat-treated palygorskite clay for methylene blue. Chem Eng J 174:143–150. https://doi.org/10.1016/J.CEJ.2011.08.062
Zhang Y, Zheng Y, Yang Y et al (2021) Mechanisms and adsorption capacities of hydrogen peroxide modified ball milled biochar for the removal of methylene blue from aqueous solutions. Bioresour Technol 337:125432. https://doi.org/10.1016/J.BIORTECH.2021.125432
Acknowledgements
The authors gratefully acknowledge the help provided by the Center of Analysis and Characterization (CAC) at Caddy Ayyad University (Marrakech, Morocco) and Ataturk University, Faculty of Engineering, Department of Metallurgical and Materials Engineering (Turkey).
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
A. Ait Baha handled all the experiments and wrote the main manuscript text. R. Idouhli and K. Tabit corrected the manuscript. O. Zakir intervened in the experimental part. B. Dikici helped with the material analysis. M. Khadiri and A. Abouelfida supervised the present work. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Not Applicable (as the results of studies do not involve any human or animal).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors state that they are clear of any financial conflicts of interest or personal ties that may have seemed to affect the research presented in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ait Baha, A., Tabit, K., Idouhli, R. et al. Zeolitization of Fumed Silica and Coal Fly Ash Using the Taguchi Method Toward Organic Pollutant Removal. Silicon 15, 6173–6184 (2023). https://doi.org/10.1007/s12633-023-02501-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12633-023-02501-8