Abstract
Introduction
The aim of this study was to describe the clinical complications, treatment use, healthcare resource utilization (HCRU), and costs among patients with sickle cell disease (SCD) with recurrent vaso-occlusive crises (VOCs) in the US.
Methods
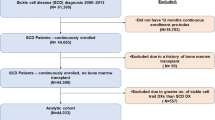
Merative MarketScan Databases were used to identify patients with SCD with recurrent VOCs from March 1, 2010, to March 1, 2019. Inclusion criteria were ≥ 1 inpatient or ≥ 2 outpatient claims for SCD and ≥ 2 VOCs per year in any 2 consecutive years after the first qualifying SCD diagnosis. Individuals without SCD in these databases were used as matched controls. Patients were followed for ≥ 12 months, from their second VOC in the 2nd year (index date) to the earliest of inpatient death, end of continuous enrollment in medical/pharmacy benefits, or March 1, 2020. Outcomes were assessed during follow-up.
Results
In total, 3420 patients with SCD with recurrent VOCs and 16,722 matched controls were identified. Patients with SCD with recurrent VOCs had a mean of 5.0 VOCs (standard deviation [SD] = 6.0), 2.7 inpatient admissions (SD 2.9), and 5.0 emergency department visits (SD 8.0) per patient per year during follow-up. Compared to matched controls, patients with SCD with recurrent VOCs incurred higher annual ($67,282 vs. $4134) and lifetime ($3.8 million vs. $229,000 over 50 years) healthcare costs.
Conclusion
Patients with SCD with recurrent VOCs experience substantial clinical and economic burden driven by inpatient costs and frequent VOCs. There is a major unmet need for treatments that alleviate or eliminate clinical complications, including VOCs, and reduce healthcare costs in this patient population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Sickle cell disease (SCD) is a hereditary hemoglobinopathy associated with substantial clinical complications, including recurrent and painful vaso-occlusive crises (VOCs) as well as high healthcare resource utilization (HCRU) |
This real-world administrative claims-based analysis represents a novel and necessary assessment of clinical and economic outcomes in the vulnerable subset of patients with SCD with recurrent VOCs |
What was learned from this study? |
Patients with SCD with recurrent VOCs experienced a mean of 5.0 VOCs (standard deviation [SD] = 6.0), 2.7 inpatient admissions (SD 2.9), and 5.0 emergency department visits (SD 8.0) per patient per year |
Compared to matched controls, patients with SCD with recurrent VOCs had significantly higher annual ($67,282 vs. $4134) and lifetime ($3.8 million vs. $229,000 over 50 years) healthcare costs, highlighting the need for cost-effective therapies that reduce clinical complications in this patient population |
Introduction
Sickle cell disease (SCD) is a hereditary hemoglobinopathy impacting ~ 100,000 individuals in the US [1, 2]. SCD is caused by a point mutation in the gene that encodes β-globin (HBB) [3]. This mutation leads to the production of abnormal or sickled hemoglobin, which polymerizes in its deoxygenated form to promote a cycle of cellular adhesion, vaso-occlusion, endothelial dysfunction, and inflammation [4, 5]. As a result of vaso-occlusion, patients with SCD experience recurrent vaso-occlusive crises (VOCs) [5, 6], the hallmark clinical feature of SCD that contributes to the development of chronic pain, multi-organ failure, and early mortality [5,6,7]. Patients with SCD also experience other serious clinical complications, including anemia, avascular necrosis, retinopathy, infection, cardiopulmonary complications, frequent hospitalizations, and poor health-related quality of life [5, 6]. Most available treatment options reduce the severity of these clinical complications but do not eliminate them [5, 6]. Currently, the only curative option is allogeneic hematopoietic stem cell transplantation; however, given the stringent requirements for donors and recipients, only a small subset of patients are eligible to receive this treatment [5, 6].
In the US, 90% of adults with SCD report having ≥ 1 severe pain event in the past 12 months [8], and up to 67% have ≥ 3 VOCs per year [9]. VOCs are the most common cause of healthcare resource utilization (HCRU) among patients with SCD, contributing to 78% of their emergency department visits and 95% of their inpatient hospital admissions [10, 11]. Moreover, suboptimal management of VOCs during an initial hospitalization is associated with frequent readmission, often as early as 1 week post-discharge [11].
Previous studies have established that VOCs lead to substantial clinical and economic burden for patients with SCD. However, these studies have largely focused on the overall patient population with SCD [28]. Ultimately, increasing access to quality healthcare for patients of all demographics is needed to minimize the clinical and economic burden of SCD, especially among Medicaid enrollees and other historically marginalized groups [26].
Several limitations in this study should be noted. First, it used administrative claims data collected for reimbursement purposes and is therefore subject to potential misclassification bias. Second, the sole reliance on direct costs in these analyses likely led to an underestimation of the economic burden of disease associated with SCD, given the significant indirect cost burden that has been reported in this population, such as negative effects on work productivity, non-work productivity, and daily activities [29, 30]. Inversely, the lifetime cost calculation used a simplifying assumption that patients with SCD will have severe disease from birth until death, which may have led to an overestimation of the actual lifetime costs in this patient population. However, our use of age-specific annual costs may have mitigated, to a degree, some of this limitation. Third, individuals who died, went on long-term disability, or did not meet eligibility criteria may have systematically different clinical outcomes than patients who met enrollment criteria. Fourth, this study did not account for any impact of recently approved therapies for SCD, such as L-glutamine, voxelotor, and crizanlizumab; therefore, pharmacy costs associated with experiencing recurrent VOCs may have been underestimated. Lastly, the results of this study may not be generalizable to patients without commercial insurance coverage, Medicare coverage, or Medicaid eligibility.
Conclusion
This study is the first to focus on clinical and economic outcomes in the subgroup of patients with SCD with recurrent VOCs, identified by having ≥ 2 VOCs per year for 2 consecutive years and using a broader composite definition for VOCs. These patients have substantial clinical complications, as well as significant HCRU and healthcare costs, largely driven by inpatient hospital admissions and the number of VOCs. Disease-modifying therapies that alleviate the clinical complications of SCD and eliminate recurrent VOCs, as well as increase access to care, are urgently needed to improve clinical and economic outcomes in this patient population.
Change history
11 September 2023
A Correction to this paper has been published: https://doi.org/10.1007/s12325-023-02629-4
References
Hassell KL. Population estimates of sickle cell disease in the US. Am J Prev Med. 2010;38:S512–21.
Hassell KL. Sickle cell disease: a continued call to action. Am J Prev Med. 2016;51:S1-2.
Finch JT, Perutz MF, Bertles JF, Dobler J. Structure of sickled erythrocytes and of sickle-cell hemoglobin fibers. Proc Natl Acad Sci USA. 1973;70:718–22.
Sundd P, Gladwin MT, Novelli EM. Pathophysiology of sickle cell disease. Annu Rev Pathol. 2019;14:263–92.
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390:311–23.
Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: definition, pathophysiology, and management. Eur J Haematol. 2020;105:237–46.
Evensen CT, Treadwell MJ, Keller S, Levine R, Hassell KL, Werner EM, et al. Quality of care in sickle cell disease: cross-sectional study and development of a measure for adults reporting on ambulatory and emergency department care. Medicine (Baltimore). 2016;95: e4528.
Zaidi AU, Glaros AK, Lee S, Wang T, Bhojwani R, Morris E, et al. A systematic literature review of frequency of vaso-occlusive crises in sickle cell disease. Orphanet J Rare Dis. 2021;16:460.
Yusuf HR, Atrash HK, Grosse SD, Parker CS, Grant AM. Emergency department visits made by patients with sickle cell disease: a descriptive study, 1999–2007. Am J Prev Med. 2010;38:S536–41.
Ballas SK, Lusardi M. Hospital readmission for adult acute sickle cell painful episodes: frequency, etiology, and prognostic significance. Am J Hematol. 2005;79:17–25.
Shah N, Bhor M, **e L, Paulose J, Yuce H. Medical resource use and costs of treating sickle cell-related vaso-occlusive crisis episodes: a retrospective claims study. J Health Econ Outcomes Res. 2020;7:52–60.
Johnson KM, Jiao B, Ramsey SD, Bender MA, Devine B, Basu A. Lifetime medical costs attributable to sickle cell disease among nonelderly individuals with commercial insurance. Blood Adv. 2023;7:365–74.
Shah NR, Bhor M, Latremouille-Viau D, Kumar Sharma V, Puckrein GA, Gagnon-Sanschagrin P, et al. Vaso-occlusive crises and costs of sickle cell disease in patients with commercial, Medicaid, and Medicare insurance - the perspective of private and public payers. J Med Econ. 2020;23:1345–55.
Desai RJ, Mahesri M, Globe D, Mutebi A, Bohn R, Achebe M, et al. Clinical outcomes and healthcare utilization in patients with sickle cell disease: a nationwide cohort study of Medicaid beneficiaries. Ann Hematol. 2020;99:2497–505.
Darbari DS, Wang Z, Kwak M, Hildesheim M, Nichols J, Allen D, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort. PLoS ONE. 2013;5(11): e79923.
Tan L, Reibman J, Ambrose C, Chung Y, Desai P, Llanos JP, et al. Clinical and economic burden of uncontrolled severe noneosinophilic asthma. Am J Manag Care. 2022;28:e212–20.
Zeiger R, Sullivan P, Chung Y, Kreindler JL, Zimmerman NM, Tkacz J. Systemic corticosteroid-related complications and costs in adults with persistent asthma. J Allergy Clin Immunol Pract. 2020;8:3455-65.e13.
Statistics USBoL. Measuring Price Change in the CPI: Medical care. Updated December 1, 2022. https://www.bls.gov/cpi/factsheets/medical-care.htm. Accessed 24 Jan 2023.
Brandow AM, Carroll CP, Creary S, Edwards-Elliott R, Glassberg J, Hurley RW, et al. American Society of Hematology 2020 guidelines for sickle cell disease: management of acute and chronic pain. Blood Adv. 2020;4:2656–701.
ClinicalTrials.gov. A Safety and Efficacy Study Evaluating CTX001 in Subjects With Severe Sickle Cell Disease. Updated December 6, 2022. https://clinicaltrials.gov/ct2/show/NCT03745287. Accessed 24 Jan 2023.
Mvundura M, Amendah D, Kavanagh PL, Sprinz PG, Grosse SD. Health care utilization and expenditures for privately and publicly insured children with sickle cell disease in the United States. Pediatr Blood Cancer. 2009;53:642–6.
Gallagher ME, Chawla A, Brady BL, Badawy SM. Heterogeneity of the long-term economic burden of severe sickle cell disease: a 5-year longitudinal analysis. J Med Econ. 2022;25:1140–8.
Millwee B, Quinn K, Goldfield N. Moving toward paying for outcomes in medicaid. J Ambul Care Manage. 2018;41:88–94.
Lopez E, Neuman T, Jacobson G, Levitt L. How much more than medicare do private insurers pay? A review of the literature. 2020. https://www.kff.org/medicare/issue-brief/how-much-more-than-medicare-do-private-insurers-pay-a-review-of-the-literature/. Accessed 15 Nov 2022.
Lee L, Smith-Whitley K, Banks S, Puckrein G. Reducing health care disparities in sickle cell disease: a review. Public Health Rep. 2019;134:599–607.
Hemker BG, Brousseau DC, Yan K, Hoffmann RG, Panepinto JA. When children with sickle-cell disease become adults: lack of outpatient care leads to increased use of the emergency department. Am J Hematol. 2011;86:863–5.
Powers-Hays A, McGann P. When actions speak louder than words—racism and sickle cell disease. N Engl J Med. 2020;383:1902–3.
Lubeck D, Agodoa I, Bhakta N, Danese M, Pappu K, Howard R, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2: e1915374.
Holdford D, Vendetti N, Sop DM, Johnson S, Smith WR. Indirect economic burden of sickle cell disease. Value Health. 2021;24:1095–101.
Acknowledgements
Funding
The study and publication fees were supported by Vertex Pharmaceuticals Incorporated and CRISPR Therapeutics AG.
Medical Writing and Editorial Assistance
The authors thank Paula J. Smith of Merative for providing programming services and Ciara Silverman, PharmD, of Vertex Pharmaceuticals Incorporated for her support in drafting this manuscript and performing data analysis. Medical writing and editing support were provided by Brittany Y. Jarrett, PhD, Natalie Prior, PhD, Jenifer Li, MSc, and Nicholas Strange of Complete HealthVizion, IPG Health Medical Communications, Inc., Chicago, IL, USA, funded by Vertex Pharmaceuticals Incorporated.
Author Contributions
All authors met the International Committee of Medical Journal Editors (ICMJE) authorship criteria. Neither honoraria nor payments were made for authorship. Chuka Udeze, Kristin A. Evans, Yoojung Yang, Timothy Lillehaugen, Janna Manjelievskaia, and Biree Andemarium were responsible for the study conception and design. Kristin A. Evans, Timothy Lillehaugen, and Janna Manjelievskaia were responsible for data acquisition. All authors participated in data analysis or interpretation, drafted or critically revised the manuscript for intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Prior Presentation
Portions of these data were presented at the Academy of Managed Care Pharmacy (AMCP) Annual Meeting 2022, National Harbor, MD, USA, October 11–14, 2022; and Society for Medical Decision Making (SMDM) 2022, Seattle, WA, USA, October 23–26, 2022.
Disclosures
Chuka Udeze, Yoojung Yang, and Nanxin Li are employees of Vertex Pharmaceuticals Incorporated and may hold stock/stock options. Urvi Mujumdar is a former employee of Vertex Pharmaceuticals Incorporated and may hold stock/stock options. Kristin A. Evans and Timothy Lillehaugen are employees of Merative and may hold stock/stock options. Janna Manjelievskaia was an employee of Merative at the time of this analysis and is now employed by Veradigm. Biree Andemarium has received research funding from the American Society of Hematology, Connecticut Department of Public Health, Forma Therapeutics, Global Blood Therapeutics, Hemanext, HRSA, Imara, Novartis, and PCORI, and has served as an advisory board member or consultant for Agios, Aruvant, Bayer, bluebird bio, CRISPR Therapeutics AG, CVS/Accordant, Cyclerion, Emmaus, Forma Therapeutics, GBT, Genentech, Hemanext, Novartis, NovoNordisk, Roche, Sanofi, TerSera, Terumo, and Vertex Pharmaceuticals Incorporated.
Compliance with Ethics Guidelines
This study employed the Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases, which include only de-identified patient data; therefore, institutional review board approval was not required.
Data Availability
This study used data available from Merative. Restrictions apply to the availability of these data, which were used under a licensing agreement.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original online version of this article was revised due to correction in Fig 2 b.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Udeze, C., Evans, K.A., Yang, Y. et al. Economic and Clinical Burden of Managing Sickle Cell Disease with Recurrent Vaso-Occlusive Crises in the United States. Adv Ther 40, 3543–3558 (2023). https://doi.org/10.1007/s12325-023-02545-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02545-7