Abstract
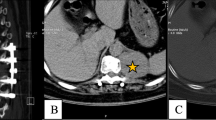
Giant cell tumor of bone (GCTB) is a rare tumor of the bone that is locally invasive. Surgery is the primary treatment that is usually done by intralesional curettage. In pelvis and spine surgery may be associated with high rate of complications, recently, Denosumab has been proposed for the treatment of these tumors in latter anatomical regions. Denosumab may be administered alone or as an adjuvant to surgery. This study aimed to assess the treatment effects of Denosumab in patients with unresectable GCTB. This study was a case series. Patients with unresectable GCTB of vertebra and sacrum were enrolled in this study. Patients received 120 mg of monthly Denosumab and additional doses on days 8th and 15th of treatment. Images of patients before and after treatment were evaluated. Nine patients with a median age of 30 years with spine and sacrum GCTB were included in this study. The median time of treatment with denosumab was 28 months (range: 3–67). Tumor control was seen in all patients. According to Inverse Choi density/size (ICDS), criteria objective response (complete response and partial response) was seen in 8 patients, and one had stable disease. Based on CT scan images, in 4 patients (44.44%), less than 50% of the transverse diameter of the tumor became ossified, and in the other five patients (55.55%), more than 50% of the tumor's transverse diameter became ossified. The median tumor volume before treatment was 829 cm3, and after treatment was 504 cm3 which was significantly reduced (P = 0.005). No complication related to therapy was seen. Tumor response was seen in all patients, and tumor control according to ICDS criteria was evident in all cases. This finding was in line with previous studies. Clinical improvement of signs and symptoms was also seen in all patients. Generally, our study demonstrates a sustained clinical benefit and tumor response with Denosumab, as tumor response ≥ 24 weeks was evident in all cases. No side effects were seen in patients despite long-term treatment with Denosumab.
Similar content being viewed by others
References
Parmeggiani A, Miceli M, Errani C, Facchini GJC (2021) State of the art and new concepts in giant cell tumor of bone: imaging features and tumor characteristics. Cancers 13(24):6298
Luther N, Bilsky MH, Härtl R (2008) Giant cell tumor of the spine. Neurosurg Clin N Am 19(1):49–55
Roessner A, Smolle M, Haybäck J (2020) Giant cell tumor of bone: Morphology, molecular pathogenesis, and differential diagnosis. Pathologe 41:134–142
Amanatullah DF, Clark TR, Lopez MJ, Borys D, Tamurian RM (2014) Giant cell tumor of bone. Orthopedics 37(2):112–120
Facchini G, Parmeggiani A, Peta G, Martella C, Gasbarrini A, Evangelisti G et al (2021) The role of percutaneous transarterial embolization in the management of spinal bone tumors: a literature review. Eur Spine J. 30(10):2839–2851
Raskin KA, Schwab JH, Mankin HJ, Springfield DS, Hornicek FJ (2013) Giant cell tumor of bone. JAAOS-J Am Acad Orthop Surg. 21(2):118–126
Siegal G, Bloem J, Cates JM (2020) WHO classification of tumors 5th Edition soft tissue and bone tumors. WHO Geneva, Switzerland
López-Pousa A, Broto JM, Garrido T, Vázquez JJC, Oncology T (2015) Giant cell tumor of bone: new treatments in development. Clin Transl Oncol. 17(6):419–430
Tariq MU, Umer M, Khan Z, Saeed J, Siddiqui MA, Din NU (2020) Spectrum of histological features of Denosumab treated giant cell tumor of bone; potential pitfalls and diagnostic challenges for pathologists. Ann Diagn Pathol. 45:151479
Wu PF, Tang JY, Li KH (2015) RANK pathway in giant cell tumor of bone: pathogenesis and therapeutic aspects. Tumor Biol. 36:495–501
Engellau J, Seeger L, Grimer R, Henshaw R, Gelderblom H, Choy E et al (2018) Assessment of denosumab treatment effects and imaging response in patients with giant cell tumor of bone. World J Surg Oncol. 16(1):1–9
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247
Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR et al (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25(13):1753–1759
Boriani S, Cecchinato R, Cuzzocrea F, Bandiera S, Gambarotti M, Gasbarrini A (2020) Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal. Eur Spine J 29:257–271
Alothman M, Althobaity W, Asiri Y, Alreshoodi S, Alismail K, Alshaalan M (2020) Giant cell tumor of bone following denosumab treatment: assessment of tumor response using various imaging modalities. Insights Imaging 11(1):1–6
Palmerini E, Chawla N, Ferrari S, Sudan M, Picci P, Marchesi E et al (2017) Denosumab in advanced/unresectable giant-cell tumour of bone (GCTB): for how long? Eur J Cancer. 76:118–124
Goldschlager T, Dea N, Boyd M, Reynolds J, Patel S, Rhines LD et al (2015) Giant cell tumors of the spine: Has denosumab changed the treatment paradigm? J Neurosurg Spine 22(5):526–533
Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay J-Y et al (2010) Denosumab in patients with giant-cell tumour of bone: an open-label phase 2 study. Lancet Oncol 11(3):275–280
Yang Y, Li Y, Liu W, Xu H, Niu XJM (2018) A nonrandomized controlled study of sacral giant cell tumors with preoperative treatment of Denosumab. Medicine. https://doi.org/10.1097/MD.0000000000013139
Branstetter DG, Nelson SD, Manivel JC, Blay J-Y, Chawla S, Thomas DM et al (2012) Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 18(16):4415–4424
Borkowska AM, Szumera-Ciećkiewicz A, Szostakowski B, Pieńkowski A, Rutkowski PL (2022) Denosumab in giant cell tumor of bone: multidisciplinary medical management based on pathophysiological mechanisms and real-world evidence. Cancers 14(9):2290
Bukata SV, Blay J-Y, Rutkowski P, Skubitz K, Henshaw R, Seeger L, Dai T, Jandial D, Chawla S (2021) Denosumab treatment for giant cell tumor of the spine including the sacrum. Spine 46(5):277
Funding
There is no funding source.
Author information
Authors and Affiliations
Contributions
AMA: made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data. MS: made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data. MC: approved the version to be published. AA: made substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data. HG: drafted the work or revised it critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human or animal participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arefpour, A., Shafieesabet, M., Chehrassan, M. et al. Effect of denosumab in treatment of unresectable spine and sacrum giant cell tumor of bone. Musculoskelet Surg 108, 93–98 (2024). https://doi.org/10.1007/s12306-023-00799-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12306-023-00799-6