Abstract
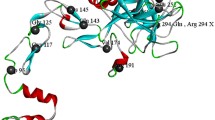
To investigate the risk factors of FVIII inhibitors development in severe hemophilia A (HA) patients who were received on-demand therapy and were infused with plasma cryoprecipitate and multiple FVIII concentrates alternately. We collected clinical information from 43 severe HA children who were treated with plasma cryoprecipitate and multiple FVIII concentrates. The F8 mutation was detected by long-distance PCR for inversion and detected by all exons and their flanking sequencing for other mutations. The inhibitor detection was performed by Nijmegen-modified Bethesda assay. The impact of novel amino substitutions on FVIII protein was predicted by SIFT and PolyPhen-2. The 3D analysis of missense mutations was performed using Swiss-PdbViewer. FVIII inhibitors were detected in nine cases (20.9%). All of the inhibitor positive cases had high risk F8 gene mutations. In most of the positive cases (7/9), inhibitors were developed during the first 10 EDs, which was significantly higher than that in the 10–50 EDs group and 50 EDs group (p = 0.009). Three novel mutations were reported, including c.214G > T (E72X), c.218 T > C (F73S), and c.2690C > G (S840X). For severe HA patients who are treated with multiple products of replacement therapy, it is important to supervise inhibitor during the first 10EDs, especially for those with high risk F8 gene mutations. F8 gene mutation is one of the most important genetic factors for inhibitor development. It is essential to detect F8 gene for all severe HA patients. Three novel mutations were reported to expand the mutation spectrum of the F8 gene.


Similar content being viewed by others
References
Mannucci PM, Tuddenham EG (2001) The hemophilias–from royal genes to gene therapy. N Engl J Med 344(23):1773–1779
Aledort L, Mannucci PM, Schramm W, Tarantino M (2019) Factor VIII replacement is still the standard of care in haemophilia A. Blood Transfus 17(6):479–486
Wight J, Paisley S (2003) The epidemiology of inhibitors in haemophilia A: a systematic review. Haemophilia 9(4):418–435
Garagiola I, Palla R, Peyvandi F (2018) Risk factors for inhibitor development in severe hemophilia A. Thromb Res 168:20–27
Bagnall RD, Waseem N, Green PM, Giannelli F (2002) Recurrent inversion breaking intron 1 of the factor VIII gene is a frequent cause of severe hemophilia A. Blood 99(1):168–174
Gouw SC, van den Berg HM, Fischer K, Auerswald G, Carcao M, Chalmers E et al (2013) Intensity of factor VIII treatment and inhibitor development in children with severe hemophilia A: the RODIN study. Blood 121(20):4046–4055
Gouw SC, van den Berg HM, Oldenburg J, Astermark J, de Groot PG, Margaglione M et al (2012) F8 gene mutation type and inhibitor development in patients with severe hemophilia A: systematic review and meta-analysis. Blood 119(12):2922–2934
Gorski MM, Blighe K, Lotta LA, Pappalardo E, Garagiola I, Mancini I et al (2016) Whole-exome sequencing to identify genetic risk variants underlying inhibitor development in severe hemophilia A patients. Blood 127(23):2924–2933
Astermark J, Donfield SM, Gomperts ED, Schwarz J, Menius ED, Pavlova A et al (2013) The polygenic nature of inhibitors in hemophilia A: results from the Hemophilia inhibitor genetics study (HIGS) combined cohort. Blood 121(8):1446–1454
Fodil M, Zemani F (2020) In silico study of correlation between missense variations of F8 gene and inhibitor formation in severe Hemophilia A. Turk J Haematol 37(2):77–83
Shinozawa K, Yada K, Kojima T, Nogami K, Taki M, Fukutake K et al (2021) Spectrum of F8 genotype and genetic impact on inhibitor development in patients with Hemophilia A from multicenter cohort studies (J-HIS) in Japan. Thromb Haemost 121(5):603–615
Peyvandi F, Cannavo A, Garagiola I, Palla R, Mannucci PM, Rosendaal FR et al (2018) Timing and severity of inhibitor development in recombinant versus plasma-derived factor VIII concentrates: a SIPPET analysis. J Thromb Haemost 16(1):39–43
Hartholt RB, van Velzen AS, Peyron I, Ten Brinke A, Fijnvandraat K, Voorberg J (2017) To serve and protect: the modulatory role of von Willebrand factor on factor VIII immunogenicity. Blood Rev 31(5):339–347
Hermans C, Astermark J, De Moerloose P (2012) Exposure to factor VIII and prediction of inhibitor development: exposure days vs. danger days, or both? J Thromb Haemost 10(10):2194–2196
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
All authors declare no competing interests and have no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Yu, Z., Zhang, W. et al. Mutation detection and inhibitor analysis of 43 children with severe hemophilia A in a single center: three novel mutations. Indian J Hematol Blood Transfus 40, 116–121 (2024). https://doi.org/10.1007/s12288-023-01675-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12288-023-01675-w