Abstract
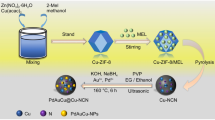
Rational construction of highly dispersed, small size, and low cost catalysts for release of hydrogen from ammonia borane (AB) is regarded as a prospective approach for promoting the development of upcoming hydrogen economy. However, the high price and scarcity of precious metal catalysts impose restrictions on their large-scale application. To this end, with the aid of a Cu doped CoZn-zeolitic imidazolate frameworks (ZIFs) template strategy, we successfully construct ultrafine monodispersed Co2P/(0.59-Cu3P) on CoZn-ZIF derived porous N-doped carbon (Co2P/(0.59-Cu3P)-NC) as an efficient non-noble-metal catalyst. Specifically, Co and Cu atoms can be geometrically separated to high degree due to the presence of Zn in the CuCoZn-ZIF precursor, and evaporation of Zn during pyrolysis can generate porous structure with the framework well maintained. The results show that porous Co2P/(0.59-Cu3P)-NC bimetallic phosphide exhibits large specific surface area, hierarchical pore structure, and well-exposed active sites. Based on the kinetics analyses and ion effects, the catalyst has achieved an unprecedentedly high total turnover frequency (TOF) of 798 \(\text{mol}_{\rm{H}_{2}}\cdot\text{mol}_{\text{cat}}^{-1}\cdot\text{min}^{-1}\) in 0.4 M NaOH solution at 298 K, which surpasses all the ever-reported transition-metal phosphides catalysts for hydrogen generation from AB. Experiments and theoretical studies confirm that the highly porous structure of the support, the ultrafine and high dispersion of nanoparticles, and the N/P do** and their synergistic effects (e.g., M-P, M-N, N-C, M-M’, and M-support) jointly induce strong electron transfer, which can reduce the reaction energy barrier and enhance their interaction with AB, thus correspondingly obtaining excellent catalytic performance. The mechanism and strategy presented in this work pave an avenue for the design of non-noble metal catalyst for hydrogen energy system.

Similar content being viewed by others
References
Huang, H. L.; Wang, C.; Li, Q.; Wang, R. Q.; Yang, Y. Y.; Muhetaer, A.; Huang, F. Q.; Han, B.; Xu, D. S. Efficient and full-spectrum photothermal dehydrogenation of ammonia borane for low-temperature release of hydrogen. Adv. Funct. Mater. 2021, 31, 2007591.
Liu, Q. B.; Zhang, S. J.; Liao, J. Y.; Feng, K. J.; Zheng, Y. Y.; Pollet, B. G.; Li, H. CuCo2O4 nanoplate film as a low-cost, highly active and durable catalyst towards the hydrolytic dehydrogenation of ammonia borane for hydrogen production. J. Power Sources 2017, 355, 191–198.
**ong, C. L.; Zhang, X. W.; Lei, Y. T.; Zhang, L. L.; Shang, H. S.; Zhang, B.; Zhao, Y. F. One-pot synthesis of ultrafine Ni0.13Co0.87P nanoparticles on halloysite nanotubes as efficient catalyst for hydrogen evolution from ammonia borane. Appl. Clay Sci. 2021, 214, 106293.
Zhang, L. J.; Ye, J.; Tu, Y.; Wang, Q. Y.; Pan, H. B.; Wu, L. H.; Zheng, X. S.; Zhu, J. F. Oxygen modified CoP2 supported palladium nanoparticles as highly efficient catalyst for hydrolysis of ammonia borane. Nano Res. 2022, 15, 3034–3041.
Feng, Y. N.; Zhou, X. P.; Yang, J. H.; Gao, X. Y.; Yin, L. X.; Zhao, Y. F.; Zhang, B. Encapsulation of ammonia borane in Pd/halloysite nanotubes for efficient thermal dehydrogenation. ACS Sustain. Chem. Eng. 2020, 8, 2122–2129.
Liu, Y.; Zhang, J.; Guan, H. J.; Zhao, Y. F.; Yang, J.-H.; Zhang, B. Preparation of bimetallic Cu-Co nanocatalysts on poly(diallyldimethylammonium chloride) functionalized halloysite nanotubes for hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2018, 427, 106–113.
Wang, J. S.; Yu, Y. Y.; Xu, W. K.; Yu, H.; Zhang, W. W.; Huang, H. L.; Zhang, G. R.; Mei, D. H. Covalent triazine framework encapsulated Pd nanoclusters for efficient hydrogen production via ammonia borane hydrolysis. J. Catal. 2022, 411, 72–83.
Yuan, C. Y.; Xu, T.; Guo, M.; Zhang, T. F.; Yu, X. B. Cation/anion-do** induced electronic structure regulation strategy to boost the catalytic hydrogen evolution from ammonia borane hydrolysis. Appl. Catal. B: Environ. 2023, 321, 122044.
Yin, L. X.; Feng, Y. N.; Zhou, X. P.; Dai, K. Q.; Gao, X. Y.; Zhao, Y. F.; Zhang, B. Synthesis of Pt nanocatalyst supported on halloysite nanotubes via strong electronic adsorption for hydrolytic dehydrogenation of ammonia borane. Chem. Lett. 2019, 48, 1084–1087.
Liu, Y. Y.; Wen, H.; Zhou, D. J.; Huang, X. Y.; Wu, X. L.; Jiang, J. C.; Guo, X. J.; Li, B. J. Tuning surface d charge of Ni-Ru alloys for unprecedented catalytic activity towards hydrogen generation from ammonia borane hydrolysis. Appl. Catal. B: Environ. 2021, 291, 120094.
Feng, Y. F.; Lv, F.; Wang, H. Z.; Chen, X. D.; Li, H.; Chen, Z. Y.; Lin, G. Y.; Liao, J. Y.; He, M. Y.; Liu, Q. B. Ni0.25Co0.75O nanowire array supported on Cu@CuO foam, an inexpensive and durable catalyst for hydrogen generation from ammonia borane. Catal. Commun. 2021, 159, 106343.
Peng, C. Y.; Kang, L.; Cao, S.; Chen, Y.; Lin, Z. S.; Fu, W. F. Nanostructured Ni2P as a robust catalyst for the hydrolytic dehydrogenation of ammonia-borane. Angew. Chem., Int. Ed. 2015, 54, 15725–15729.
Zhao, L. Q.; Wei, Q. H.; Zhang, L. L.; Zhao, Y. F.; Zhang, B. NiCo alloy decorated on porous N-doped carbon derived from ZnCo-ZIF as highly efficient and magnetically recyclable catalyst for hydrogen evolution from ammonia borane. Renew. Energy 2021, 173, 273–282.
Yao, Q. L.; Lu, Z. H.; Huang, W.; Chen, X. S.; Zhu, J. High Pt-like activity of the Ni-Mo/graphene catalyst for hydrogen evolution from hydrolysis of ammonia borane. J. Mater. Chem. A 2016, 4, 8579–8583.
Xu, Q.; Chandra, M. Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia-borane at room temperature. J. Power Sources 2006, 163, 364–370.
Feng, K.; Zhong, J.; Zhao, B. H.; Zhang, H.; Xu, L.; Sun, X. H.; Lee, S. T. CuxCo1−xO nanoparticles on graphene oxide as a synergistic catalyst for high-efficiency hydrolysis of ammonia-borane. Angew. Chem., Int. Ed. 2016, 55, 11950–11954.
Han, A.; Chen, H. L.; Zhang, H. Y.; Sun, Z. J.; Du, P. W. Ternary metal phosphide nanosheets as a highly efficient electrocatalyst for water reduction to hydrogen over a wide pH range from 0 to 14. J. Mater. Chem. A 2016, 4, 10195–10202.
Nguyen, M. T.; Wakabayashi, R. H.; Yang, M. H.; Abruña, H. D.; DiSalvo, F. J. Synthesis of carbon supported ordered tetragonal pseudo-ternary Pt2M′M″ (M = Fe, Co, Ni) nanoparticles and their activity for oxygen reduction reaction. J. Power Sources 2015, 280, 459–466.
Grosvenor, A. P.; Cavell, R. G.; Mar, A. Next-nearest neighbour contributions to P 2p3/2 X-ray photoelectron binding energy shifts of mixed transition-metal phosphides M1−xM′xP with the MnP-type structure. J. Solid State Chem. 2007, 180, 2702–2712.
Guan, S. Y.; Zhang, L. N.; Zhang, H. H.; Guo, Y.; Liu, B. Z.; Wen, H.; Fan, Y. P.; Li, B. J. Defect-rich Co-CoOx-graphene nanocatalysts for efficient hydrogen production from ammonia borane. Chem.—Asian J. 2020, 15, 3087–3095.
Li, H. J.; Yao, Z.; Wang, X. T.; Zhu, Y. L.; Chen, Y. R. Review on hydrogen production from catalytic ammonia borane methanolysis: Advances and perspectives. Energy Fuel. 2022, 36, 11745–11759.
Lu, D. S.; Li, J. H.; Lin, C. H.; Liao, J. Y.; Feng, Y. F.; Ding, Z. T.; Li, Z. W.; Liu, Q. B.; Li, H. A simple and scalable route to synthesize CoxCu1−xCo2O4@CoyCu1−yCo2O4 yolk—shell microspheres, a high-performance catalyst to hydrolyze ammonia borane for hydrogen production. Small 2019, 15, 1805460.
Zhang, L.; Fu, W. Q.; Yu, Q. Y.; Tang, T. D.; Zhao, Y. C.; Li, Y. D. Effect of citric acid addition on the morphology and activity of Ni2P supported on mesoporous zeolite ZSM-5 for the hydrogenation of 4, 6-DMDBT and phenanthrene. J. Catal. 2017, 345, 295–307.
Qu, X. P.; Jiang, R.; Li, Q.; Zeng, F. N.; Zheng, X.; Xu, Z. M.; Chen, C. H.; Peng, J. The hydrolysis of ammonia borane catalyzed by NiCoP/OPC-300 nanocatalysts: High selectivity and efficiency, and mechanism. Green Chem. 2019, 21, 850–860.
Peng, W.; Yang, X. X.; Mao, L. C.; **, J. H.; Yang, S. L.; Zhang, J. J.; Li, G. ZIF-67-derived Co nanoparticles anchored in N doped hollow carbon nanofibers as bifunctional oxygen electrocatalysts. Chem. Eng. J. 2021, 407, 127157.
Wei, Q. H.; Qiu, S. J.; Yin, C. W.; Liu, J. X.; **a, Y. P.; Wen, X.; Zou, Y. J.; Xu, F.; Sun, L. X.; Chu, H. L. Nitrogen-doped carbon encapsulated Ru-decorated Co2P supported on graphene oxide as efficient catalysts for hydrogen generation from ammonia borane. J. Alloys Compd. 2022, 921, 166207.
Aksoy, M.; Korkut, S. E.; Metin, Ö. AuPt alloy nanoparticles supported on graphitic carbon nitride: In situ synthesis and superb catalytic performance in the light-assisted hydrolytic dehydrogenation of ammonia borane. Appl. Surf. Sci. 2022, 602, 154286.
Liang, H. W.; Brüller, S.; Dong, R. H.; Zhang, J.; Feng, X. L.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992.
Cui, K. X.; Zhong, W. F.; Li, L. Y.; Zhuang, Z. Y.; Li, L. Y.; Bi, J. H.; Yu, Y. Well-defined metal nanoparticles@covalent organic framework yolk—shell nanocages by ZIF-8 template as catalytic nanoreactors. Small 2019, 15, 1804419.
Li, F.; Bu, Y. F.; Lv, Z. J.; Mahmood, J.; Han, G. F.; Ahmad, I.; Kim, G.; Zhong, Q.; Baek, J. B. Porous cobalt phosphide polyhedrons with iron do** as an efficient bifunctional electrocatalyst. Small 2017, 13, 1701167.
Liao, J. Y.; Shao, Y. X.; Feng, Y. F.; Zhang, J.; Song, C. X.; Zeng, W.; Tang, J. T.; Dong, H. F.; Liu, Q. B.; Li, H. Interfacial charge transfer induced dual-active-sites of heterostructured Cu0.8Ni0.2WO4 nanoparticles in ammonia borane methanolysis for fast hydrogen production. Appl. Catal. B: Environ. 2023, 320, 121973.
Yang, Y. J.; Ma, Q. X.; Han, L.; Tao, K. Zeolitic imidazolate framework-derived Co3S4@Co(OH)2 nanoarrays as self-supported electrodes for asymmetric supercapacitors. Inorg. Chem. Front. 2019, 6, 1398–1404.
Peng, Z.; Yu, Y.; Jiang, D.; Wu, Y. L.; **a, B. Y.; Dong, Z. H. N-doped carbon shell coated CoP nanocrystals encapsulated in porous N-doped carbon substrate as efficient electrocatalyst of water splitting. Carbon 2019, 144, 464–471.
Hou, C. C.; Chen, Q. Q.; Li, K.; Wang, C. J.; Peng, C. Y.; Shi, R.; Chen, Y. Tailoring three-dimensional porous cobalt phosphides templated from bimetallic metal-organic frameworks as precious metal-free catalysts towards the dehydrogenation of ammonia-borane. J. Mater. Chem. A 2019, 7, 8277–8283.
Liu, S. H.; Li, J.; Yan, X.; Su, Q. F.; Lu, Y. H.; Qiu, J. S.; Wang, Z. Y.; Lin, X. D.; Huang, J. L.; Liu, R. L. et al. Superhierarchical cobalt-embedded nitrogen-doped porous carbon nanosheets as two-in-one hosts for high-performance lithium-sulfur batteries. Adv. Mater. 2018, 30, 1706895.
Yuan, Y.; Chen, X. Y.; Zhang, X.; Wang, Z. M.; Yu, R. B. A MOF-derived CuCo(O)@carbon-nitrogen framework as an efficient synergistic catalyst for the hydrolysis of ammonia borane. Inorg. Chem. Front. 2020, 7, 2043–2049.
Guo, L.; Bai, X.; Xue, H.; Sun, J.; Song, T. S.; Zhang, S.; Qin, L.; Huang, K. K.; He, F.; Wang, Q. MOF-derived hierarchical 3D Bi-doped CoP nanoflower eletrocatalyst for hydrogen evolution reaction in both acidic and alkaline media. Chem. Commun. 2020, 56, 7702–7705.
Gong, Y. Q.; Zhong, H.; Liu, W. H.; Zhang, B. B.; Hu, S. Q.; Wang, R. H. General synthetic route toward highly dispersed ultrafine Pd-Au alloy nanoparticles enabled by imidazolium-based organic polymers. ACS Appl. Mater. Interfaces 2018, 10, 776–786.
Wang, C. L.; Tuninetti, J.; Wang, Z.; Zhang, C.; Ciganda, R.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of ammonia-borane over Ni/ZIF-8 nanocatalyst: High efficiency, mechanism, and controlled hydrogen release. J. Am. Chem. Soc. 2017, 139, 11610–11615.
Li, X. Y.; Jiang, Q. Q.; Dou, S.; Deng, L. B.; Huo, J.; Wang, S. Y. ZIF-67-derived Co-NC@CoP-NC nanopolyhedra as an efficient bifunctional oxygen electrocatalyst. J. Mater. Chem. A 2016, 4, 15836–15840.
Diao, L. C.; Yang, T.; Chen, B.; Zhang, B.; Zhao, N. Q.; Shi, C. S.; Liu, E. Z.; Ma, L. Y.; He, C. N. Electronic reconfiguration of Co2P induced by Cu do** enhancing oxygen reduction reaction activity in zinc-air batteries. J. Mater. Chem. A 2019, 7, 21232–21243.
Zhang, H.; Yang, Z.; Wang, X. M.; Yan, S. H.; Zhou, T. Y.; Zhang, C.; Telfer, S. G.; Liu, S. X. Uniform copper-cobalt phosphides embedded in N-doped carbon frameworks as efficient bifunctional oxygen electrocatalysts for rechargeable Zn-air batteries. Nanoscale 2019, 11, 17384–17395.
Wei, Z. H.; Liu, Y.; Peng, Z. K.; Song, H. Q.; Liu, Z. Y.; Liu, B. Z.; Li, B. J.; Yang, B.; Lu, S. Y. Cobalt-ruthenium nanoalloys parceled in porous nitrogen-doped graphene as highly efficient difunctional catalysts for hydrogen evolution reaction and hydrolysis of ammonia borane. ACS Sustain. Chem. Eng. 2019, 7, 7014–7023.
Zhou, H. P.; Hu, J. Facile synthesis of multi-walled carbon nanotubes/Co9S8 composites with enhanced performances for sodium-ion battery. Mater. Lett. 2017, 195, 26–30.
Cheng, J.; Yang, X.; Xuan, X. X.; Liu, N.; Zhou, J. H. Efficient conversion of carbon dioxide on atomically dispersed metal-Nx species-anchored porous carbon with embedded CuxCoy nanoparticles by accelerating electron separation. ACS Sustain. Chem. Eng. 2020, 8, 5994–6002.
Wen, L. L.; Sun, Y. Q.; Zhang, C.; Yu, J.; Li, X. Y.; Lyu, X. J.; Cai, W. P.; Li, Y. Cu-doped CoP nanorod arrays: Efficient and durable hydrogen evolution reaction electrocatalysts at all pH values. ACS Appl. Energy Mater. 2018, 1, 3835–3842.
Guan, S. Y.; An, L. L.; Ashraf, S.; Zhang, L. N.; Liu, B. Z.; Fan, Y. P.; Li, B. J. Oxygen vacancy excites Co3O4 nanocrystals embedded into carbon nitride for accelerated hydrogen generation. Appl. Catal. B: Environ. 2020, 269, 118775.
Kuang, M.; Wang, Q. H.; Han, P.; Zheng, G. F. Cu, Co-embedded N-enriched mesoporous carbon for efficient oxygen reduction and hydrogen evolution reactions. Adv. Energy Mater. 2017, 7, 1700193.
Hou, J. G.; Sun, Y. Q.; Wu, Y. Z.; Cao, S. Y.; Sun, L. C. Promoting active sites in core—shell nanowire array as Mott—Schottky electrocatalysts for efficient and stable overall water splitting. Adv. Funct. Mater. 2018, 28, 1704447.
Park, Y. S.; Choi, W. S.; Jang, M. J.; Lee, J. H.; Park, S.; **, H.; Seo, M. H.; Lee, K. H.; Yin, Y. D.; Kim, Y. et al. Three-dimensional dendritic Cu-Co-P electrode by one-step electrodeposition on a hydrogen bubble template for hydrogen evolution reaction. ACS Sustain. Chem. Eng. 2019, 7, 10734–10741.
Wang, R.; Dong, X. Y.; Du, J.; Zhao, J. Y.; Zang, S. Q. MOF-derived bifunctional Cu3P nanoparticles coated by a N,P-codoped carbon shell for hydrogen evolution and oxygen reduction. Adv. Mater. 2018, 30, 1703711.
Luo, X. H.; Zhou, Q. L.; Du, S.; Li, J.; Zhang, L.; Lin, K. D.; Li, H.; Chen, B.; Wu, T.; Chen, D. C. et al. One-dimensional porous hybrid structure of Mo2C-CoP encapsulated in N-doped carbon derived from MOF: An efficient electrocatalyst for hydrogen evolution reaction over the entire pH range. ACS Appl. Mater. Interfaces 2018, 10, 42335–42347.
Han, X. P.; Ling, X. F.; Wang, Y.; Ma, T. Y.; Zhong, C.; Hu, W. B.; Deng, Y. D. Generation of nanoparticle, atomic-cluster, and single-atom cobalt catalysts from zeolitic imidazole frameworks by spatial isolation and their use in zinc-air batteries. Angew. Chem., Int. Ed. 2019, 58, 5359–5364.
Lai, S. J.; Xu, L.; Liu, H. L.; Chen, S.; Cai, R. S.; Zhang, L. J.; Theis, W.; Sun, J.; Yang, D. J.; Zhao, X. L. Controllable synthesis of CoN3 catalysts derived from Co/Zn-ZIF-67 for electrocatalytic oxygen reduction in acidic electrolytes. J. Mater. Chem. A 2019, 7, 21884–21891.
Yin, L. X.; Zhang, T. T.; Dai, K. Q.; Zhang, B.; **ang, X.; Shang, H. S. Ultrafine PtCo alloy nanoclusters confined in N-doped mesoporous carbon spheres for efficient ammonia borane hydrolysis. ACS Sustain. Chem. Eng. 2021, 9, 822–832.
Fu, F. Y.; Wang, C. L.; Wang, Q.; Martinez-Villacorta, A. M.; Escobar, A.; Chong, H. B.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E. et al. Highly selective and sharp volcano-type synergistic Ni2Pt@ZIF-8-catalyzed hydrogen evolution from ammonia borane hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042.
Wang, Q.; Fu, F. Y.; Yang, S.; Moro, M. M.; de los Angeles Ramirez, M.; Moya, S.; Salmon, L.; Ruiz, J.; Astruc, D. Dramatic synergy in CoPt nanocatalysts stabilized by “click” dendrimers for evolution of hydrogen from hydrolysis of ammonia borane. ACS Catal. 2019, 9, 1110–1119.
Chen, W. Y.; Li, D. L.; Peng, C.; Qian, G.; Duan, X. Z.; Chen, D.; Zhou, X. G. Mechanistic and kinetic insights into the Pt-Ru synergy during hydrogen generation from ammonia borane over PtRu/CNT nanocatalysts. J. Catal. 2017, 356, 186–196.
Xu, S. H.; Wang, J. F.; Guo, J. P.; Zhang, W. Y.; de Souza, A. A. U.; He, D. N. Activating Co nanoparticles on P-doped carbon nitride via enhancing Mott—Schottky effect by constructing interfacial chemical bonding for the efficient dehydrogenation of ammonia-borane. Appl. Surf. Sci. 2020, 533, 146999.
Basu, S.; Brockman, A.; Gagare, P.; Zheng, Y.; Ramachandran, P. V.; Delgass, W. N.; Gore, J. P. Chemical kinetics of Ru-catalyzed ammonia borane hydrolysis. J. Power Sources 2009, 188, 238–243.
Li, Y. T.; Zhang, X. L.; Peng, Z. K.; Liu, P.; Zheng, X. C. Hierarchical porous g-C3N4 coupled ultrafine RuNi alloys as extremely active catalysts for the hydrolytic dehydrogenation of ammonia borane. ACS Sustain. Chem. Eng. 2020, 8, 8458–8468.
Ge, Y. Z.; Qin, X. T.; Li, A. W.; Deng, Y. C.; Lin, L. L.; Zhang, M. T.; Yu, Q. L.; Li, S. W.; Peng, M.; Xu, Y. et al. Maximizing the synergistic effect of CoNi catalyst on α-MoC for robust hydrogen production. J. Am. Chem. Soc. 2021, 143, 628–633.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. U20041100 and 21706242) and Science and Technology Project of Henan Province (No. 212102110068). The authors really appreciate Modern Analysis and Gene Sequencing Center of Zhengzhou University in help of characterization.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2023_5463_MOESM1_ESM.pdf
Constructing ultrafine monodispersed Co2P/(0.59-Cu3P) on Cu doped CoZn-ZIF derived porous N-doped carbon for highly efficient dehydrogenation of ammonia borane
Rights and permissions
About this article
Cite this article
Yang, Y., Zhao, L., Gao, X. et al. Constructing ultrafine monodispersed Co2P/(0.59-Cu3P) on Cu doped CoZn-ZIF derived porous N-doped carbon for highly efficient dehydrogenation of ammonia borane. Nano Res. 16, 6687–6700 (2023). https://doi.org/10.1007/s12274-023-5463-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5463-y