Abstract
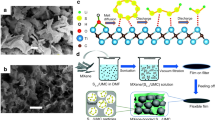
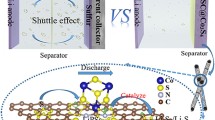
Lithium-sulfur batteries are regarded as promising next-generation energy storage batteries for their ultra-high theoretical energy density. However, the complex sulfur electrode process with sluggish sulfur conversion reactions is a critical issue for lithium-sulfur batteries, in which catalytic interfacial reactions and accelerated lithium-ion diffusion are the key factors. Our previous work has shown that implanting functional molecules with multiple redox properties in the electrode can break through the conventional diffusion layer constraints and achieve forced convection. In this work, a functionalized complex molecule, methylene blue anthraquinone-2-sulfonate (MB-AQ), with multiple redox activities as well as abundant active sites, was synthesized and introduced into the sulfur cathode. In addition to accelerating the transport of lithium ions by reversible inhaling and exhaling lithium ions, the MB-AQ can combine polysulfides by its active sites to accelerate sulfur conversion reactions. Benefiting from two functions of accelerating ion diffusion and catalyzing interfacial reactions, MB-AQ/reduced graphene oxide (rGO)/S cathode can achieve high initial capacities of 884 and 674 mAh·g−1 with stable cycling of 700 and 1,000 times at 1 and 4 C, respectively. It is worth mentioning that the capacity of 462 mAh·g-1 can be achieved even at a high current density of 6 C. This work provides a new approach to enhancing the sulfur cathode process.

Similar content being viewed by others
References
Manthiram, A.; Chung, S. H.; Zu, C. X. Lithium-sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006.
Dunn, B.; Kamath, H.; Tarascon, J. M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935.
Seh, Z. W.; Sun, Y. M.; Zhang, Q. F.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634.
Song, Y. Z.; Cai, W. L.; Kong, L.; Cai, J. S.; Zhang, Q.; Sun, J. Y. Rationalizing electrocatalysis of Li-S chemistry by mediator design: Progress and prospects. Adv. Energy Mater. 2020, 10, 1901075.
Bonnick, P.; Muldoon, J. The Dr Jekyll and Mr Hyde of lithium sulfur batteries. Energy Environ. Sci. 2020, 13, 4808–4833.
Zhao, C. X.; Li, X. Y.; Zhao, M.; Chen, Z. X.; Song, Y. W.; Chen, W. J.; Liu, J. N.; Wang, B.; Zhang, X. Q.; Chen, C. M. et al. Semi-immobilized molecular electrocatalysts for high-performance lithium-sulfur batteries. J. Am. Chem. Soc. 2021, 143, 19865–19872.
Yang, B.; Guo, D. Y.; Lin, P. R.; Zhou, L.; Li, J.; Fang, G. Y.; Wang, J. Y.; **, H. L.; Chen, X. A.; Wang, S. Hydroxylated multi-walled carbon nanotubes covalently modified with tris(hydroxypropyl) phosphine as a functional interlayer for advanced lithium-sulfur batteries. Angew. Chem., Int. Ed. 2022, 61, e202204327.
Yang, D. W.; Zhang, C. Q.; Biendicho, J. J.; Han, X.; Liang, Z. F.; Du, R. F.; Li, M. Y.; Li, J. S.; Arbiol, J.; Llorca, J. et al. ZnSe/N-doped carbon nanoreactor with multiple adsorption sites for stable lithium-sulfur batteries. ACS Nano 2020, 14, 15492–15504.
Liu, J. Y.; Ding, Y. Y.; Shen, Z. H.; Zhang, H. G.; Han, T. L.; Guan, Y.; Tian, Y. C.; Braun, P. V. A Lamellar yolk—shell lithium-sulfur battery cathode displaying ultralong cycling life, high rate performance, and temperature tolerance. Adv. Sci (Weinh.) 2022, 9, 2103517.
Fei, B.; Zhang, C. Q.; Cai, D. P.; Zheng, J. Y.; Chen, Q. D.; **e, Y. L.; Zhu, L. Z.; Cabot, A.; Zhan, H. B. Hierarchical nanoreactor with multiple adsorption and catalytic sites for robust lithium-sulfur batteries. ACS Nano 2021, 15, 6849–6860.
Lei, J.; Fan, X. X.; Liu, T.; Xu, P.; Hou, Q.; Li, K.; Yuan, R. M.; Zheng, M. S.; Dong, Q. F.; Chen, J. J. Single-dispersed polyoxometalate clusters embedded on multilayer graphene as a bifunctional electrocatalyst for efficient Li-S batteries. Nat. Commun. 2022, 13, 202.
Du, Z. Z.; Chen, X. J.; Hu, W.; Chuang, C. H.; **e, S.; Hu, A. J.; Yan, W. S.; Kong, X. H.; Wu, X. J.; Ji, H. X. et al. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium-sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985.
Li, C.; **, Z.; Guo, D.; Chen, X.; Yin, L. Chemical immobilization effect on lithium polysulfides for lithium-sulfur batteries. Small 2018, 14, 1701986.
Wang, P.; Sun, F. H.; **ong, S. L.; Zhang, Z. C. Y.; Duan, B.; Zhang, C. H.; Feng, J. K.; **, B. J. WSe2 flakelets on N-doped graphene for accelerating polysulfide redox and regulating Li plating. Angew. Chem., Int. Ed. 2022, 61, e202116048.
Zhou, G. M.; Tian, H. Z.; **, Y.; Tao, X. Y.; Liu, B. F.; Zhang, R. F.; Seh, Z. W.; Zhuo, D.; Liu, Y. Y.; Sun, J. et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li-S batteries. Proc. Natl. Acad. Sci. USA 2017, 114, 840–845.
Fan, X. X.; Lei, J.; Hou, Q.; Lin, X. D.; Xu, P.; Fan, J. M.; Yuan, R. M.; Zheng, M. S.; Dong, Q. F. Forced ion flux by multi-redox molecule to break diffusion limit and boost electrode process. Cell Rep. Phys. Sci. 2022, 3, 100826.
Wang, Z. J.; Fan, Q. Q.; Guo, W.; Yang, C. C.; Fu, Y. Z. Biredox-ionic anthraquinone-coupled ethylviologen composite enables reversible multielectron redox chemistry for Li-organic batteries. Adv. Sci. 2022, 9, 2103632.
Faul, C. F. J.; Antonietti, M. Ionic self-assembly: Facile synthesis of supramolecular materials. Adv. Mater. 2003, 15, 673–683.
Franke, D.; Vos, M.; Antonietti, M.; Sommerdijk, N. A. J. M.; Faul, C. F. J. Induced supramolecular chirality in nanostructured materials: Ionic self-assembly of perylene-chiral surfactant complexes. Chem. Mater. 2006, 18, 1839–1847.
Martin, K. E.; Wang, Z. C.; Busani, T.; Garcia, R. M.; Chen, Z.; Jiang, Y. B.; Song, Y. J.; Jacobsen, J. L.; Vu, T. T.; Schore, N. E. Donor—acceptor biomorphs from the ionic self-assembly of porphyrins. J. Am. Chem. Soc. 2010, 132, 8194–8201.
Wei, Z. X.; Shin, W.; Jiang, H.; Wu, X. Y.; Stickle, W. F.; Chen, G.; Lu, J.; Alex Greaney, P.; Du, F.; Ji, X. L. Reversible intercalation of methyl viologen as a dicationic charge carrier in aqueous batteries. Nat. Commun. 2019, 10, 3227.
Luo, M. S.; Li, S.; Di, Z. X.; Yang, Z.; Chou, W. C.; Shi, B. C. Fischer—Tropsch synthesis: Effect of nitric acid pretreatment on graphene-supported cobalt catalyst. Appl. Catal. A:Gen. 2020, 599, 117608.
Molina, A.; Patil, N.; Ventosa, E.; Liras, M.; Palma, J.; Marcilla, R. New anthraquinone-based conjugated microporous polymer cathode with ultrahigh specific surface area for high-performance lithium-ion batteries. Adv. Funct. Mater. 2020, 30, 1908074.
Zhu, C. Y.; Zhang, W. J.; Li, G.; Li, C. L.; Qin, X. H. Ultra-simple and green two-step synthesis of sodium anthraquinone-2-sulfonate composite graphene (AQS/rGO) hydrogels for supercapacitor electrode materials. J. Alloys Compd. 2021, 862, 158472.
Ovchinnikov, O. V.; Evtukhova, A. V.; Kondratenko, T. S.; Smirnov, M. S.; Khokhlov, V. Y.; Erina, O. V. Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib. Spectrosc. 2016, 86, 181–189.
Zhang, Y. D.; An, Y. F.; Wu, L. Y.; Chen, H.; Li, Z. H.; Dou, H.; Murugadoss, V.; Fan, J. C.; Zhang, X. G.; Mai, X. M. et al. Metal-free energy storage systems: Combining batteries with capacitors based on a methylene blue functionalized graphene cathode. J. Mater. Chem. A 2019, 7, 19668–19675.
Hernández, G.; Işik, M.; Mantione, D.; Pendashteh, A.; Navalpotro, P.; Shanmukaraj, D.; Marcilla, R.; Mecerreyes, D. Redox-active poly(ionic liquid)s as active materials for energy storage applications. J. Mater. Chem. A 2017, 5, 16231–16240.
Zhao, J.; Yang, J. X.; Sun, P. F.; Xu, Y. H. Sodium sulfonate groups substituted anthraquinone as an organic cathode for potassium batteries. Electrochem. Commun. 2018, 86, 34–37.
Yang, J.; Yang, Y.; Li, A. R.; Wang, Z. C.; Wang, H.; Yu, D. D.; Hu, P. F.; Qian, M. M.; Lin, J.; Guo, L. Sustainable treatment of dye wastewater for high-performance rechargeable battery cathodes. Energy Stor. Mater. 2019, 17, 334–340.
Kosswattaarachchi, A. M.; Cook, T. R. Repurposing the industrial dye methylene blue as an active component for redox flow batteries. ChemElectroChem 2018, 5, 3437–3442.
Dai, Q. P.; Zhang, J. F.; Ma, M. The formation of composites from imidazolate polymer with epoxy resins. Appl. Surf. Sci. 1993, 72, 67–72.
Yamada, Y.; Kim, J.; Matsuo, S.; Sato, S. Nitrogen-containing graphene analyzed by X-ray photoelectron spectroscopy. Carbon 2014, 70, 59–74.
Seh, Z. W.; Wang, H. T.; Liu, N.; Zheng, G. Y.; Li, W. Y.; Yao, H. B.; Cui, Y. High-capacity Li2S-graphene oxide composite cathodes with stable cycling performance. Chem. Sci. 2014, 5, 1396–1400.
Fu, Y. S.; Wu, Z.; Yuan, Y. F.; Chen, P.; Yu, L.; Yuan, L.; Han, Q. R.; Lan, Y. J.; Bai, W. X.; Kan, E. J. et al. Switchable encapsulation of polysulfides in the transition between sulfur and lithium sulfide. Nat. Commun. 2020, 11, 845.
Chen, W.; Qian, T.; **ong, J.; Xu, N.; Liu, X. J.; Liu, J.; Zhou, J. Q.; Shen, X. W.; Yang, T. Z.; Chen, Y. et al. A new type of multifunctional polar binder: Toward practical application of high energy lithium sulfur batteries. Adv. Mater. 2017, 29, 1605160.
Zhang, B. H.; Wu, J. F.; Gu, J. K.; Li, S.; Yan, T. Y.; Gao, X. P. The fundamental understanding of lithium polysulfides in ether-based electrolyte for lithium-sulfur batteries. ACS Energy Lett. 2021, 6, 537–546.
Zhang, G.; Peng, H. J.; Zhao, C. Z.; Chen, X.; Zhao, L. D.; Li, P.; Huang, J. Q.; Zhang, Q. The radical pathway based on a lithium-metal-compatible high-dielectric electrolyte for lithium-sulfur batteries. Angew. Chem., Int. Ed. 2018, 57, 16732–16736.
Moy, D.; Manivannan, A.; Narayanan, S. R. Direct measurement of polysulfide shuttle current: A window into understanding the performance of lithium-sulfur cells. J. Electrochem. Soc. 2015, 162, A1–A7.
Peng, H. J.; Zhang, Z. W.; Huang, J. Q.; Zhang, G.; **e, J.; Xu, W. T.; Shi, J. L.; Chen, X.; Cheng, X. B.; Zhang, Q. A cooperative interface for highly efficient lithium-sulfur batteries. Adv. Mater. 2016, 28, 9551–9558.
Tan, G. Q.; Xu, R.; **ng, Z. Y.; Yuan, Y. F.; Lu, J.; Wen, J. G.; Liu, C.; Ma, L.; Zhan, C.; Liu, Q. et al. Burning lithium in CS2 for high-performing compact Li2S-graphene nanocapsules for Li-S batteries. Nat. Energy 2017, 2, 17090.
Deng, Z. F.; Zhang, Z.; Lai, Y. Q.; Liu, J.; Li, J.; Liu, Y. X. Electrochemical impedance spectroscopy study of a lithium/sulfur battery: Modeling and analysis of capacity fading. J. Electrochem. Soc. 2013, 160, A553–A558.
Sadd, M.; De Angelis, S.; Colding-Jørgensen, S.; Blanchard, D.; Johnsen, R. E.; Sanna, S.; Borisova, E.; Matic, A.; Bowen, J. R. Visualization of dissolution—precipitation processes in lithium-sulfur batteries. Adv. Energy Mater. 2022, 12, 2103126.
Zhang, C. Q.; Du, R. F.; Biendicho, J. J.; Yi, M. J.; **ao, K.; Yang, D. W.; Zhang, T.; Wang, X.; Arbiol, J.; Llorca, J. et al. Tubular CoFeP@CN as a Mott—Schottky catalyst with multiple adsorption sites for robust lithium-sulfur batteries. Adv. Energy Mater. 2021, 11, 2100432.
Yuan, H. D.; Chen, X. L.; Zhou, G. M.; Zhang, W. K.; Luo, J. M.; Huang, H.; Gan, Y. P.; Liang, C.; **a, Y.; Zhang, J. et al. Efficient activation of Li2S by transition metal phosphides nanoparticles for highly stable lithium-sulfur batteries. ACS Energy Lett. 2017, 2, 1711–1719.
Yuan, H. D.; Zhang, W. K.; Wang, J. G.; Zhou, G. M.; Zhuang, Z. Z.; Luo, J. M.; Huang, H.; Gan, Y. P.; Liang, C.; **a, Y. et al. Facilitation of sulfur evolution reaction by pyridinic nitrogen doped carbon nanoflakes for highly-stable lithium-sulfur batteries. Energy Stor. Mater. 2018, 10, 1–9.
Lin, Y. L.; Huang, S.; Zhong, L.; Wang, S. J.; Han, D. M.; Ren, S.; **ao, M.; Meng, Y. Z. Organic liquid electrolytes in Li-S batteries: Actualities and perspectives. Energy Stor. Mater. 2021, 34, 128–147.
Zhang, S. S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. U1805254, 21773192, 22072117, and 22179112).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Zheng, Q., Fan, X., Liu, G. et al. Enhancing sulfur cathode process via a functionalized complex molecule. Nano Res. 16, 8385–8393 (2023). https://doi.org/10.1007/s12274-022-5282-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-5282-6