Abstract
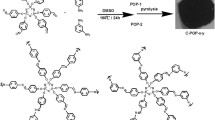
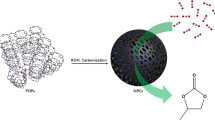
Replacing traditional polymer-based precursors with small molecules is a promising pathway toward facile and controllable preparation of porous carbons but remains a prohibitive challenge because of the high volatility of small molecules. Herein, a simple, general, and controllable method is reported to prepare porous carbons by converting small organic molecules into organic molecular salts followed by pyrolysis. The robust electrostatic force holding organic molecular salts together leads to negligible volatility and thus ensures the formation of carbons under high-temperature pyrolysis. Meanwhile, metal moieties in organic molecular salts can be evolved into in-situ templates or activators during pyrolysis to create nanopores. The modular nature of organic molecular salts allows easy control of the porosity and chemical do** of carbons at a molecular level. The sulfur-doped carbon prepared by the ionic solid strategy can serve as robust support to prepare small-sized intermetallic PtCo catalysts, which exhibit a high mass activity of 1.62 A·mgPt−1 in catalyzing oxygen reduction reaction for fuel cell applications.

Similar content being viewed by others
References
Zhai, Y. P.; Dou, Y. Q.; Zhao, D. Y.; Fulvio, P. F.; Mayes, R. T.; Dai, S. Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850.
Su, D. S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816.
Borchardt, L.; Zhu, Q. L.; Casco, M. E.; Berger, R.; Zhuang, X. D.; Kaskel, S.; Feng, X. L.; Xu, Q. Toward a molecular design of porous carbon materials. Mater. Today 2017, 20, 592–610.
Benzigar, M. R.; Talapaneni, S. N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721.
Paraknowitsch, J. P.; Thomas, A. Do** carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855.
Perovic, M.; Qin, Q.; Oschatz, M. From molecular precursors to nanoparticles—Tailoring the adsorption properties of porous carbon materials by controlled chemical functionalization. Adv. Funct. Mater. 2020, 30, 1908371.
Stein, A.; Wang, Z. Y.; Fierke, M. A. Functionalization of porous carbon materials with designed pore architecture. Adv. Mater. 2009, 21, 265–293.
Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280.
Dutta, S.; Bhaumik, A.; Wu, K. C. W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592.
Wang, H.; Shao, Y.; Mei, S. L.; Lu, Y.; Zhang, M.; Sun, J. K.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Y. Polymer-derived heteroatom-doped porous carbon materials. Chem. Rev. 2020, 120, 9363–9419.
Ito, Y.; Christodoulou, C.; Nardi, M. V.; Koch, N.; Kläui, M.; Sachdev, H.; Müllen, K. Tuning the magnetic properties of carbon by nitrogen do** of its graphene domains. J. Am. Chem. Soc. 2015, 137, 7678–7685.
Chen, Z. P.; Ren, W. C.; Gao, L. B.; Liu, B. L.; Pei, S. F.; Cheng, H. M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428.
Chang, Y. Q.; Antonietti, M.; Fellinger, T. P. Synthesis of nanostructured carbon through ionothermal carbonization of common organic solvents and solutions. Angew. Chem., Int. Ed. 2015, 54, 5507–5512.
Lee, J. S.; Wang, X. Q.; Luo, H. M.; Baker, G. A.; Dai, S. Facile ionothermal synthesis of microporous and mesoporous carbons from task specific ionic liquids. J. Am. Chem. Soc. 2009, 131, 4596–4597.
Wang, X. Q.; Dai, S. Ionic liquids as versatile precursors for functionalized porous carbon and carbon-oxide composite materials by confined carbonization. Angew. Chem. 2010, 122, 6814–6818.
Paraknowitsch, J. P.; Zhang, J.; Su, D. S.; Thomas, A.; Antonietti, M. Ionic liquids as precursors for nitrogen-doped graphitic carbon. Adv. Mater. 2010, 22, 87–92.
Fellinger, T. P.; Thomas, A.; Yuan, J. Y.; Antonietti, M. 25th anniversary article: “cooking carbon with salt”: Carbon materials and carbonaceous frameworks from ionic liquids and poly(ionic liquid)s. Adv. Mater. 2013, 25, 5838–5855.
Zhang, S. G.; Miran, M. S.; Ikoma, A.; Dokko, K.; Watanabe, M. Protic ionic liquids and salts as versatile carbon precursors. J. Am. Chem. Soc. 2014, 136, 1690–1693.
Zhang, S. G.; Dokko, K.; Watanabe, M. Direct synthesis of nitrogen-doped carbon materials from protic ionic liquids and protic salts: Structural and physicochemical correlations between precursor and carbon. Chem. Mater. 2014, 26, 2915–2926.
Zhang, S. G.; Tsuzuki, S.; Ueno, K.; Dokko, K.; Watanabe, M. Upper limit of nitrogen content in carbon materials. Angew. Chem., Int. Ed. 2015, 54, 1302–1306.
Wu, Z. Y.; Xu, S. L.; Yan, Q. Q.; Chen, Z. Q.; Ding, Y. W.; Li, C.; Liang, H. W.; Yu, S. H. Transition metal-assisted carbonization of small organic molecules toward functional carbon materials. Sci. Adv. 2018, 4, eaat0788.
Liang, H. W.; Brüller, S.; Dong, R. H.; Zhang, J.; Feng, X. L.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992.
Lewis, I. C. Chemistry of carbonization. Carbon 1982, 20, 519–529.
Sevilla, M.; Díez, N.; Fuertes, A. B. More sustainable chemical activation strategies for the production of porous carbons. ChemSusChem 2021, 14, 94–117.
Wang, C. H.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J. S.; Yamauchi, Y. New strategies for novel MOF-derived carbon materials based on nanoarchitectures. Chem 2020, 6, 19–40.
Tong, L.; Zhang, L. L.; Wang, Y. C.; Wan, L. Y.; Yan, Q. Q.; Hua, C.; Jiao, C. J.; Zhou, Z. Y.; Ding, Y. W.; Liu, B. et al. Hierarchically porous carbons derived from nonporous coordination polymers. ACS Appl. Mater. Interfaces 2020, 12, 25211–25220.
Hussain, T.; Nie, P. F.; Hu, B.; Shang, X. H.; Yang, J. M.; Li, J. Y. Facile synthesis of Mg-formate MOF-derived mesoporous carbon for fast capacitive deionization. J. Mater. Sci. 2021, 56, 10282–10292.
Luo, M. C.; Zhao, Z. L.; Zhang, Y. L.; Sun, Y. J.; **ng, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y. N. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 2019, 574, 81–85.
Lin, F. X.; Lv, F.; Zhang, Q. H.; Luo, H.; Wang, K.; Zhou, J. H.; Zhang, W. Y.; Zhang, W. S.; Wang, D. W.; Gu, L. et al. Local coordination regulation through tuning atomic-scale cavities of Pd metallene toward efficient oxygen reduction electrocatalysis. Adv. Mater. 2022, 34, 2202084.
Wang, C. Y.; Spendelow, J. S. Recent developments in Pt-Co catalysts for proton-exchange membrane fuel cells. Curr. Opin. Electrochem. 2021, 28, 100715.
Yan, Y. C.; Du, J. S.; Gilroy, K. D.; Yang, D. R.; **a, Y. N.; Zhang, H. Intermetallic nanocrystals: Syntheses and catalytic applications. Adv. Mater. 2017, 29, 1605997.
Wang, D. L.; **n, H. L.; Hovden, R.; Wang, H. S.; Yu, Y. C.; Muller, D. A.; DiSalvo, F. J.; Abruna, H. D. Structurally ordered intermetallic platinum-cobalt core—shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87.
Yang, C. L.; Wang, L. N.; Yin, P.; Liu, J. Y.; Chen, M. X.; Yan, Q. Q.; Wang, Z. S.; Xu, S. L.; Chu, S. Q.; Cui, C. H. et al. Sulfur-anchoring synthesis of platinum intermetallic nanoparticle catalysts for fuel cells. Science 2021, 374, 459–464.
Yin, P.; Luo, X.; Ma, Y. F.; Chu, S. Q.; Chen, S.; Zheng, X. S.; Lu, J. L.; Wu, X. J.; Liang, H. W. Sulfur stabilizing metal nanoclusters on carbon at high temperatures. Nat. Commun. 2021, 12, 3135.
Kitchin, J. R.; Nørskov, J. K.; Barteau, M. A.; Chen, J. C. Modification of the surface electronic and chemical properties of Pt (111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246.
Mavrikakis, M.; Hammer, B.; Nørskov, J. K. Effect of strain on the reactivity of metal surfaces. Phys. Rev. Lett. 1998, 81, 2819–2822.
Antolini, E. Alloy vs. intermetallic compounds: Effect of the ordering on the electrocatalytic activity for oxygen reduction and the stability of low temperature fuel cell catalysts. Appl. Catal. B Environ. 2017, 217, 201–213.
Han, B. H.; Carlton, C. E.; Kongkanand, A.; Kukreja, R. S.; Theobald, B. R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F. T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266.
Li, J. R.; Sharma, S.; Liu, X. M.; Pan, Y. T.; Spendelow, J. S.; Chi, M. F.; Jia, Y. K.; Zhang, P.; Cullen, D. A.; **, Z. et al. Hard-magnet L10-CoPt nanoparticles advance fuel cell catalysis. Joule 2019, 3, 124–135.
Acknowledgements
We acknowledge the funding support from the National Key Research and Development Program of China (No. 2018YFA0702001), the National Natural Science Foundation of China (No. 22071225), the Fundamental Research Funds for the Central Universities (No. WK2060190103), the Joint Funds from Hefei National Synchrotron Radiation Laboratory (No. KY2060000175), the Natural Science Foundation of Guangdong Province (No. 2021A1515012356), the Research Grant for Scientific Platform and Project of Guangdong Provincial Education office (No. 2019KTSCX151), Shenzhen Government’s Plan of Science and Technology (No. JCYJ20180305125247308), and the Collaborative Innovation Program of Hefei Science Center of CAS (No. 2021HSC-CIP015). L. D. F. acknowledges the support from the Instrumental Analysis Center of Shenzhen University (**li Campus).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Tong, L., Yang, QQ., Li, S. et al. Building the bridge of small organic molecules to porous carbons via ionic solid principle. Nano Res. 16, 80–87 (2023). https://doi.org/10.1007/s12274-022-4997-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4997-8