Abstract
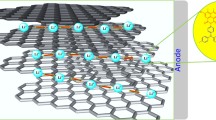
As a novel class of porous crystalline solids, covalent organic frameworks (COFs) based electrolyte can combine the advantages of both inorganic and polymer electrolytes, leading to such as higher structural stability to inhibit lithium dendrites and better processing facility for improving interfacial contact. However, the ionic components of Li salt tend to be closely associated in the form of ion pairs or even ionic aggregates in the channel of COFs due to strong coulombic interactions, thus resulting in slow ionic diffusion dynamics and low ionic conductivity. Herein, we successfully designed and synthesized a novel single-ion conducting nitrogen hybrid conjugated skeleton (NCS) as all solid electrolyte, whose backbone is consisted with triazine and piperazine rings. A loose bonding between the triazine rings and cations would lower the energy barrier during ions transfer, and electrostatic forces with piperazine rings could “anchor” anions to increase the selectivity during ions transfer. Thus, the NCS-electrolyte exhibits excellent room temperature lithium-ion conductivity up to 1.49 mS·cm−1 and high transference number of 0.84 without employing any solvent, which to the best of our knowledge is one of the highest COF-based electrolytes so far. Moreover, the fabricated all-solid-state lithium metal batteries demonstrate highly attractive properties with quite stable cycling performance over 100 cycles with 82% capacity reservation at 0.5 C.

Similar content being viewed by others
References
Duffner, F.; Kronemeyer, N.; Tübke, J.; Leker, J.; Winter, M.; Schmuch, R. Post-lithium-ion battery cell production and its compatibility with lithium-ion cell production infrastructure. Nat. Energy 2021, 6, 123–134.
Yang, Y. N.; Jiang, F. L.; Li, Y. Q.; Wang, Z. X.; Zhang, T. A surface coordination interphase stabilizes a solid-state battery. Angew. Chem., Int. Ed. 2021, 60, 24162–24170.
Pan, K. C.; Zhang, L.; Qian, W. W.; Wu, X. K.; Dong, K.; Zhang, H. T.; Zhang, S. J. A flexible ceramic/polymer hybrid solid electrolyte for solid-state lithium metal batteries. Adv. Mater. 2020, 32, e2000399.
Ji, X.; Hou, S.; Wang, P. F.; He, X. Z.; Piao, N.; Chen, J.; Fan, X. L.; Wang, C. S. Solid-state electrolyte design for lithium dendrite suppression. Adv. Mater. 2020, 32, e2002741.
Shi, K.; Wan, Z. P.; Yang, L.; Zhang, Y. W.; Huang, Y. F.; Su, S. M.; **a, H. Y.; Jiang, K. L.; Shen, L.; Hu, Y. et al. In situ construction of an ultra-stable conductive composite interface for high-voltage all-solid-state lithium metal batteries. Angew. Chem. Int. Ed. 2020, 59, 11784–11788.
Niu, C. Q.; Luo, W. J.; Dai, C. M.; Yu, C. B.; Xu, Y. X. High-voltage-tolerant covalent organic framework electrolyte with holistically oriented channels for solid-state lithium metal batteries with nickel-rich cathodes. Angew. Chem., Int. Ed. 2021, 60, 24915–24923.
Vazquez-Molina, D. A.; Mohammad-Pour, G. S.; Lee, C.; Logan, M. W.; Duan, X. F.; Harper, J. K.; Uribe-Romo, F. J. Mechanically shaped two-dimensional covalent organic frameworks reveal crystallographic alignment and fast Li-ion conductivity. J. Am. Chem. Soc. 2016, 138, 9767–9770.
Chen, Z.; Zhang, H. R.; Dong, T. T.; Mu, P. Z.; Rong, X. C.; Li, Z. T. Uncovering the chemistry of cross-linked polymer binders via chemical bonds for silicon-based electrodes. ACS Appl. Mater. Interfaces 2020, 12, 47164–47180.
Du, Y.; Yang, H. S.; Whiteley, J. M.; Wan, S.; **, Y. H.; Lee, S. H.; Zhang, W. Ionic covalent organic frameworks with spiroborate linkage. Angew. Chem., Int. Ed. 2016, 55, 1737–1741.
Hu, Y. M.; Dunlap, N.; Wan, S.; Lu, S. L.; Huang, S. F.; Sellinger, I.; Ortiz, M.; **, Y. H.; Lee, S. H.; Zhang, W. Crystalline lithium imidazolate covalent organic frameworks with high Li-ion conductivity. J. Am. Chem. Soc. 2019, 141, 7518–7525.
Jeong, K.; Park, S.; Jung, G. Y.; Kim, S. H.; Lee, Y. H.; Kwak, S. K.; Lee, S. Y. Solvent-free, single lithium-ion conducting covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 5880–5885.
Li, X.; Hou, Q.; Huang, W.; Xu, H. S.; Wang, X. W.; Yu, W.; Li, R. L.; Zhang, K.; Wang, L.; Chen, Z. X. et al. Solution-processable covalent organic framework electrolytes for all-solid-state Li-organic batteries. ACS Energy Lett. 2020, 5, 3498–3506.
He, X. F.; Zhu, Y. Z.; Mo, Y. F. Origin of fast ion diffusion in superionic conductors. Nat. Commun. 2017, 8, 15893.
Cheng, Z. Y.; **e, M. L.; Mao, Y. Y.; Ou, J. X.; Zhang, S. J.; Zhao, Z.; Li, J. L.; Fu, F.; Wu, J. H.; Shen, Y. B. et al. Building lithiophilic ion-conduction highways on garnet-type solid-state Li+ conductors. Adv. Energy Mater. 2020, 10, 1904230.
Liang, J. Y.; Zeng, X. X.; Zhang, X. D.; Zuo, T. T.; Yan, M.; Yin, Y. X.; Shi, J. L.; Wu, X. W.; Guo, Y. G.; Wan, L. J. Engineering Janus interfaces of ceramic electrolyte via distinct functional polymers for stable high-voltage Li-metal batteries. J. Am. Chem. Soc. 2019, 141, 9165–9169.
Kasemchainan, J.; Zekoll, S.; Spencer Jolly, D.; Ning, Z. Y.; Hartley, G. O.; Marrow, J.; Bruce, P. G. Critical strip** current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 2019, 18, 1105–1111.
Xu, S. J.; Sun, Z. H.; Sun, C. G.; Li, F.; Chen, K.; Zhang, Z. H.; Hou, G. J.; Cheng, H. M.; Li, F. Homogeneous and fast ion conduction of PEO-based solid-state electrolyte at low temperature. Adv. Funct. Mater. 2020, 30, 2007172.
Zhao, Q.; Liu, X. T.; Stalin, S.; Khan, K.; Archer, L. A. Solid-state polymer electrolytes with in-built fast interfacial transport for secondary lithium batteries. Nat. Energy 2019, 4, 365–373.
Zhang, H.; Li, C. M.; Piszcz, M.; Coya, E.; Rojo, T.; Rodriguez-Martinez, L. M.; Armand, M.; Zhou, Z. B. Single lithium-ion conducting solid polymer electrolytes: Advances and perspectives. Chem. Soc. Rev. 2017, 46, 797–815.
Ashraf, S.; Zuo, Y. M.; Li, S.; Liu, C. X.; Wang, H.; Feng, X.; Li, P. F.; Wang, B. Crystalline anionic germanate covalent organic framework for high CO2 selectivity and fast Li ion conduction. Chem. -Eur. J. 2019, 25, 13479–13483.
Li, Z.; Liu, Z. W.; Li, Z. Y.; Wang, T. X.; Zhao, F. L.; Ding, X. S.; Feng, W.; Han, B. H. Defective 2D covalent organic frameworks for postfunctionalization. Adv. Funct. Mater. 2020, 30, 1909267.
Xu, Q.; Tao, S. S.; Jiang, Q. H.; Jiang, D. L. Ion conduction in polyelectrolyte covalent organic frameworks. J. Am. Chem. Soc. 2018, 140, 7429–7432.
Zhang, Y. Y.; Duan, J. Y.; Ma, D.; Li, P. F.; Li, S. W.; Li, H. W.; Zhou, J. W.; Ma, X. J.; Feng, X.; Wang, B. Three-dimensional anionic cyclodextrin-based covalent organic frameworks. Angew. Chem., Int. Ed. 2017, 56, 16313–16317.
Li, X. R.; Tian, Y.; Shen, L.; Qu, Z. B.; Ma, T. Q.; Sun, F.; Liu, X. Y.; Zhang, C.; Shen, J. Q.; Li, X. Y. et al. Electrolyte interphase built from anionic covalent organic frameworks for lithium dendrite suppression. Adv. Funct. Mater. 2021, 31, 2009718.
Chen, H. W.; Tu, H. Y.; Hu, C. J.; Liu, Y.; Dong, D. R.; Sun, Y. F.; Dai, Y. F.; Wang, S. L.; Qian, H.; Lin, Z. Y. et al. Cationic covalent organic framework nanosheets for fast Li-ion conduction. J. Am. Chem. Soc. 2018, 140, 896–899.
Schmidtchen, F. P.; Berger, M. Artificial organic host molecules for anions. Chem. Rev. 1997, 97, 1609–1646.
Wiers, B. M.; Foo, M. L.; Balsara, N. P.; Long, J. R. A solid lithium electrolyte via addition of lithium isopropoxide to a metal-organic framework with open metal sites. J. Am. Chem. Soc. 2011, 133, 14522–14525.
Fujie, K.; Otsubo, K.; Ikeda, R.; Yamada, T.; Kitagawa, H. Low temperature ionic conductor: Ionic liquid incorporated within a metal-organic framework. Chem. Sci. 2015, 6, 4306–4310.
Guo, Z. B.; Zhang, Y. Y.; Dong, Y.; Li, J.; Li, S. W.; Shao, P. P.; Feng, X.; Wang, B. Fast ion transport pathway provided by polyethylene glycol confined in covalent organic frameworks. J. Am. Chem. Soc. 2019, 141, 1923–1927.
Zhang, G.; Hong, Y. L.; Nishiyama, Y.; Bai, S. Y.; Kitagawa, S.; Horike, S. Accumulation of glassy poly(ethylene oxide) anchored in a covalent organic framework as a solid-state Li+ electrolyte. J. Am. Chem. Soc. 2019, 141, 1227–1234.
Zhao, Z. D.; Chen, W. J.; Impeng, S.; Li, M. X.; Wang, R.; Liu, Y. C.; Zhang, L.; Dong, L.; Unruangsri, J.; Peng, C. X. et al. Covalent organic framework-based ultrathin crystalline porous film: Manipulating uniformity of fluoride distribution for stabilizing lithium metal anode. J. Mater. Chem. A 2020, 8, 3459–3467.
Feng, X.; Ding, X. S.; Jiang, D. L. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022.
Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M. V.; Heine, T.; Banerjee, R. Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J. Am. Chem. Soc. 2012, 134, 19524–19527.
Nagai, A.; Guo, Z. Q.; Feng, X.; **, S. B.; Chen, X.; Ding, X. S.; Jiang, D. L. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536.
Skorjanc, T.; Shetty, D.; Olson, M. A.; Trabolsi, A. Design strategies and redox-dependent applications of insoluble viologen-based covalent organic polymers. ACS Appl. Mater. Interfaces 2019, 11, 6705–6716.
Yan, Y. C.; Chen, Z.; Yang, J.; Guan, L.; Hu, H.; Zhao, Q. S.; Ren, H.; Lin, Y.; Li, Z. T.; Wu, M. B. Controllable substitution of S radicals on triazine covalent framework to expedite degradation of polysulfides. Small 2020, 16, e2004631.
Zhao, H. Y.; **, Z.; Su, H. M.; **g, X. F.; Sun, F. X.; Zhu, G. S. Targeted synthesis of a 2D ordered porous organic framework for drug release. Chem. Commun. (Camb.) 2011, 47, 6389–6391.
Cinar, H.; Kretschmann, O.; Ritter, H. Synthesis of novel fluorinated polymers via cyclodextrin complexes in aqueous solution. Macromolecules 2005, 38, 5078–5082.
Fang, R. Y.; Xu, B. Y.; Grundish, N. S.; **a, Y.; Li, Y. T.; Lu, C. W.; Liu, Y. J.; Wu, N.; Goodenough, J. B. Li2S6-integrated PEO-based polymer electrolytes for all-solid-state lithium-metal batteries. Angew. Chem., Int. Ed. 2021, 60, 17701–17706.
Wu, N.; Chien, P. H.; Li, Y. T.; Dolocan, A.; Xu, H. H.; Xu, B. Y.; Grundish, N. S.; **, H. B.; Hu, Y. Y.; Goodenough, J. B. Fast Li+ conduction mechanism and interfacial chemistry of a NASICON/polymer composite electrolyte. J. Am. Chem. Soc. 2020, 142, 2497–2505.
Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the dam** function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465.
Acknowledgements
We thank the financial support from the Natural Science Foundation of Shandong (Nos. ZR2020JQ21 and ZR2021ZD24), National Natural Science Foundation of China (Nos. 51873231 and 22138013), Taishan Scholar Project (No. tsqn201909062), and the Technology Foundation of Shandong Energy Group Co., LTD. (YKZB2020-176, YKKJ2019AJ08JG-R63). This work was partially carried out at the City University of Hong Kong for MD simulations. We greatly thank Pro. Jian LU give us assistance on NMR data unscrambling and analyzing.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Cheng, Z., Lu, L., Zhang, S. et al. Amphoteric covalent organic framework as single Li+ superionic conductor in all-solid-state. Nano Res. 16, 528–535 (2023). https://doi.org/10.1007/s12274-022-4783-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4783-7