Abstract
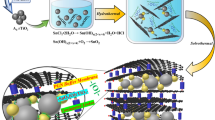
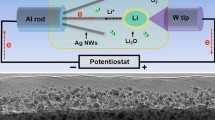
Rechargeable lithium-carbon dioxide (Li-CO2) batteries have attracted much attention due to their high theoretical energy densities and capture of CO2. However, the electrochemical reaction mechanisms of rechargeable Li-CO2 batteries, particularly the decomposition mechanisms of the discharge product Li2CO3 are still unclear, impeding their practical applications. Exploring electrochemistry of Li2CO3 is critical for improving the performance of Li-CO2 batteries. Herein, in-situ environmental transmission electron microscopy (ETEM) technique was used to study electrochemistry of Li2CO3 in Li-CO2 batteries during discharge and charge processes. During discharge, Li2CO3 was nucleated and accumulated on the surface of the cathode media such as carbon nanotubes (CNTs) and Ag nanowires (Ag NWs), but it was hard to decompose during charging at room temperature. To promote the decomposition of Li2CO3, the charge reactions were conducted at high temperatures, during which Li2CO3 was decomposed to lithium with release of gases. Density functional theory (DFT) calculations revealed that the synergistic effect of temperature and biasing facilitates the decomposition of Li2CO3. This study not only provides a fundamental understanding to the high temperature Li-CO2 nanobatteries, but also offers a valid technique, i.e., discharging/charging at high temperatures, to improve the cyclability of Li-CO2 batteries for energy storage applications.

Similar content being viewed by others
References
Xu, S. M.; Das, S. K.; Archer, L. A. The Li-CO2 battery: A novel method for CO2 capture and utilization. RSC Adv. 2013, 3, 6656–6660.
Ahmadiparidari, A.; Warburton, R. E.; Majidi, L.; Asadi, M.; Chamaani, A.; Jokisaari, J. R.; Rastegar, S.; Hemmat, Z.; Sayahpour, B.; Assary, R. S. et al. A long-cycle-life lithium-CO2 battery with carbon neutrality. Adv. Mater. 2019, 31, 1902518.
Li, C.; Guo, Z. Y.; Yang, B. C.; Liu, Y.; Wang, Y. G.; **a, Y. Y. A rechargeable Li-CO2 battery with a gel polymer electrolyte. Angew. Chem., Int. Ed. 2017, 56, 9126–9130.
Wang, Y.; Wang, W. W.; **e, J.; Wang, C. H.; Yang, Y. W.; Lu Y. C. Electrochemical reduction of CO2 in ionic liquid: Mechanistic study of Li-CO2 batteries via in situ ambient pressure X-ray photoelectron spectroscopy. Nano Energy 2021, 83: 10583
Feng, N. N.; Wang, B. L.; Yu, Z.; Gu, Y. M.; Xu L. L.; Ma, J.; Wang, Y. G.; **a, Y. Y. Mechanism-of-action elucidation of reversible Li-CO2 batteries using the water-in-salt electrolyte. ACS Appl. Mater. Interfaces 2021, 13, 7396–7404.
Zhang, B. W.; Jiao, Y.; Chao, D. L.; Ye, C.; Wang, Y. X.; Davey, K.; Liu, H. K.; Dou, S. X.; Qiao, S. Z. Targeted synergy between adjacent Co atoms on graphene oxide as an efficient new electrocatalyst for Li-CO2 batteries. Adv. Funct. Mater. 2019, 29, 1904206.
Hou, Y. Y.; Wang, J. Z.; Liu, L. L.; Liu, Y. Q.; Chou, S. L.; Shi, D. Q.; Liu, H. K.; Wu, Y. P.; Zhang, W. M.; Chen, J. Mo2C/CNT: An efficient catalyst for rechargeable Li-CO2 batteries. Adv. Funct. Mater. 2017, 27, 1700564.
**ao, Y.; Du, F.; Hu, C. G.; Ding, Y.; Wang, Z. L.; Roy, A.; Dai, L. M. High-performance Li-CO2 batteries from free-standing, binder-free, bifunctional three-dimensional carbon catalysts. ACS Energy Lett. 2020, 5, 916–921.
Zhang, X.; Wang, C. Y.; Li, H. H.; Wang, X. G.; Chen, Y. N.; **e, Z. J.; Zhou, Z. High performance Li-CO2 batteries with NiO-CNT cathodes. J. Mater. Chem. A 2018, 6, 2792–2796.
Lu, S. Y.; Shang, Y.; Ma, S. Y.; Lu, Y. C.; Liu, Q. C.; Li, Z. J. Porous NiO nanofibers as an efficient electrocatalyst towards long cycling life rechargeable Li-CO2 batteries. Electrochim. Acta 2019, 319, 958–965.
Song, S. D.; Xu, W.; Zheng, J. M.; Luo, L. L.; Engelhard, M. H.; Bowden M. E.; Liu, B.; Wang, C. M.; Zhang, J. G. Complete decomposition of Li2CO3 in Li-O2 batteries using Ir/B4C as noncarbon-based oxygen electrode. Nano Lett. 2017, 17, 1417–1424.
Pipes, P.; Bhargav, A.; Manthiram, A. Phenyl disulfide additive for solution-mediated carbon dioxide utilization in Li-CO2 batteries. Adv. Energy Mater. 2019, 9, 1900453.
Yin, W.; Grimaud, A.; Azcarate, I.; Yang, C. Z.; Tarascon, J. M. Electrochemical reduction of CO2 mediated by quinone derivatives: Implication for Li-CO2 battery. J. Phys. Chem. C 2018, 122, 6546–6554.
Khurram, A.; He, M. F.; Gallant, B. M. Tailoring the discharge reaction in Li-CO2 batteries through incorporation of CO2 capture chemistry. Joule 2018, 2, 2649–2666.
Oh, D.; Qi, J. F.; Lu, Y. C.; Zhang, Y.; Yang, S. H.; Belcher, A. M. Biologically enhanced cathode design for improved capacity and cycle life for lithium-oxygen batteries. Nat. Commun. 2013, 4, 2756.
Das, S. K.; Xu, S. M.; Emwas, A. H.; Lu, Y. Y.; Srivastava, S.; Archer, L. A. High energy lithium-oxygen batteries-transport barriers and thermodynamics. Energy Environ. Sci. 2012, 5, 8927–8931.
Yang, S. X.; Qiao, Y.; He, P.; Liu, Y. J.; Cheng, Z.; Zhu, J. J.; Zhou, H. S. A reversible lithium-CO2 battery with Ru nanoparticles as a cathode catalyst. Energy Environ. Sci. 2017, 10, 972–978.
Wang, C. Y.; Zhang, Q. M.; Zhang, X.; Wang, X. G.; **e, Z. J.; Zhou, Z. Fabricating Ir/C nanofiber networks as free-standing air cathodes for rechargeable Li-CO2 batteries. Small 2018, 14, 1800641.
Hou, Y. H.; Erni, R.; Widmer, R.; Rahaman, M.; Guo, H. Z.; Fasel, R.; Moreno-García, P.; Zhang, Y. C.; Broekmann, P. Synthesis and characterization of degradation-resistant Cu@CuPd nanowire catalysts for the efficient production of formate and CO from CO2. ChemElectroChem 2019, 6, 3189–3198.
Kuang, M.; Guan, A. X.; Gu, Z. X.; Han, P.; Qian, L. P.; Zheng, G. F. Enhanced N-do** in mesoporous carbon for efficient electrocatalytic CO2 conversion. Nano Res. 2019, 12, 2324–2329.
Zhang, Z.; Ma, C.; Tu, Y. C.; Si, R.; Wei, J.; Zhang, S. H.; Wang, Z.; Li, J. F.; Wang, Y.; Deng, D. H. Multiscale carbon foam confining single iron atoms for efficient electrocatalytic CO2 reduction to CO. Nano Res. 2019, 12, 2313–2317.
Guo, Z. Y.; Li, J. L.; Qi, H. C.; Sun, X. M.; Li, H. D.; Tamirat, A. G.; Liu, J.; Wang, Y. G.; Wang, L. A highly reversible long-life Li-CO2 battery with a RuP2-based catalytic cathode. Small 2019, 15, 1803246.
Wang, L. J.; Dai, W. R.; Ma, L. P.; Gong, L. L.; Lyu, Z. Y.; Zhou, Y.; Liu, J.; Lin, M.; Lai, M.; Peng, Z. Q. et al. Monodispersed Ru nanoparticles functionalized graphene nanosheets as efficient cathode catalysts for O2-assisted Li-CO2 battery. ACS Omega 2017, 2, 9280–9286.
Bie, S. Y.; Du, M. L.; He, W. X.; Zhang, H. G.; Yu, Z. T.; Liu, J. G.; Liu, M.; Yan, W. W.; Zhou, L.; Zou, Z. G. Carbon nanotube@RuO2 as a high performance catalyst for Li-CO2 batteries. ACS Appl. Mater. Interfaces 2019, 11, 5146–5151.
Yang, C.; Guo, K. K.; Yuan, D. W.; Cheng, J. L.; Wang, B. Unraveling reaction mechanisms of Mo2C as cathode catalyst in a Li-CO2 battery. J. Am. Chem. Soc. 2020, 142, 6983–6990.
Jena, A.; Hsieh, H. C.; Thoka, S.; Hu, S. F.; Chang, H.; Liu, R. S. Curtailing the overpotential of Li-CO2 batteries with shape-controlled Cu2O as cathode: Effect of illuminating the cathode. ChemSusChem 2020, 13, 2719–2725.
Ma, W. Q.; Lu, S. S.; Lei, X. F.; Liu, X. Z.; Ding, Y. Porous Mn2O3 cathode for highly durable Li-CO2 batteries. J. Mater. Chem. A 2018, 6, 20829–20835.
Thoka, S.; Chen, C. J.; Jena, A.; Wang, F. M.; Wang, X. C.; Chang, H.; Hu, S. F.; Liu, R. S. Spinel zinc cobalt oxide (ZnCo2O4) porous nanorods as a cathode material for highly durable Li-CO2 batteries. ACS Appl. Mater. Interfaces 2020, 12, 17353–17363.
Zhang, P. F.; Zhang, J. Y.; Sheng, T.; Lu, Y. Q.; Yin, Z. W.; Li, Y. Y.; Peng, X. X.; Zhou, Y.; Li, J. T.; Wu, Y. J. et al. Synergetic effect of Ru and NiO in the electrocatalytic decomposition of Li2CO3 to enhance the performance of a Li-CO2/O2 battery. ACS Catal. 2020, 10, 1640–1651.
Zhang, Z.; Zhang, Q.; Chen, Y. N.; Bao, J.; Zhou, X. L.; **e, Z. J.; Wei, J. P.; Zhou, Z. The first introduction of graphene to rechargeable Li-CO2 batteries. Angew. Chem., Int. Ed. 2015, 54, 6550–6553.
Qiao, Y.; Liu, Y.; Chen, C. J.; **e, H.; Yao, Y. G.; He, S. M.; **, W. W.; Liu, B. Y.; Hu, L. B. 3D-printed graphene oxide framework with thermal shock synthesized nanoparticles for Li-CO2 batteries. Adv. Funct. Mater. 2018, 28, 1805899.
Zhang, X.; Zhang, Q.; Zhang, Z.; Chen, Y. N.; **e, Z. J.; Wei, J. P.; Zhou, Z. Rechargeable Li-CO2 batteries with carbon nanotubes as air cathodes. Chem. Commun. 2015, 51, 14636–14639.
Yang, H.; Wang, M.; Liu, X. W.; Jiang, Y.; Yu, Y. MoS2 embedded in 3D interconnected carbon nanofiber film as a free-standing anode for sodium-ion batteries. Nano Res. 2018, 11, 3844–3853.
Song, T.; Han, H.; Choi, H.; Lee, J. W.; Park, H.; Lee, S.; Park, W. I.; Kim, S.; Liu, L.; Paik, U. TiO2 nanotube branched tree on a carbon nanofiber nanostructure as an anode for high energy and power lithium ion batteries. Nano Res. 2014, 7, 491–501.
Li, S. W.; Liu, Y.; Zhou, J. W.; Hong, S. S.; Dong, Y.; Wang, J. M.; Gao, X.; Qi, P. F.; Han, Y. Z.; Wang, B. Monodispersed MnO nanoparticles in graphene—An interconnected N-doped 3D carbon framework as a highly efficient gas cathode in Li-CO2 batteries. Energy Environ. Sci. 2019, 12, 1046–1054.
Wang, X. F.; Liu, H. X.; Wang, Q.; Huo, J. H.; Ge, W. Y.; Duan, X. B.; Guo, S. W. Microbial-derived functional carbon decorated hollow NiCo-LDHs nanoflowers as a highly efficient catalyst for Li-CO2 battery. Appl. Surf. Sci. 2021, 540, 148351.
Guan, D. H.; Wang, X. X.; Li, M. L.; Li, F.; Zheng, L. J.; Huang, X. L.; Xu, J. J. Light/electricity energy conversion and storage for a hierarchical porous In2S3@CNT/SS cathode towards a flexible Li-CO2 battery. Angew. Chem., Int. Ed. 2020, 59, 19518–19524.
Hu, X. F.; Sun, J. C.; Li, Z. F.; Zhao, Q.; Chen, C. C.; Chen, J. Rechargeable room-temperature Na-CO2 batteries. Angew. Chem., Int. Ed. 2016, 55, 6482–6486.
Hu, X. F.; Li, Z. F.; Chen, J. Flexible Li-CO2 batteries with liquid-free electrolyte. Angew. Chem., Int. Ed. 2017, 56, 5785–5789.
**e, J. F.; Liu, Q.; Huang, Y. Y.; Wu, M. X.; Wang, Y. B. A porous Zn cathode for Li-CO2 batteries generating fuel-gas CO. J. Mater. Chem. A 2018, 6, 13952–13958.
Zhao, Z. W.; Wang, E. K.; Wang, J. W.; Liu, C. T.; Peng, Z. Q. Kinetics of the CO2 reduction reaction in aprotic Li-CO2 batteries: A model study. J. Mater. Chem. A 2021, 9, 3290–3296.
Zhao, Z. W.; Su, Y. W.; Peng, Z. Q. Probing lithium carbonate formation in trace-O2-assisted aprotic Li-CO2 batteries using in situ surface-enhanced Raman spectroscopy. J. Phys. Chem. Lett. 2019, 10, 322–328.
Qiao, Y.; Yi, J.; Wu, S. C.; Liu, Y.; Yang, S. X.; He, P.; Zhou, H. S. Li-CO2 electrochemistry: A new strategy for CO2 fixation and energy storage. Joule 2017, 1, 359–370.
Saito, Y.; Matsumoto, T.; Nishikubo, K. Encapsulation of carbides of chromium, molybdenum and tungsten in carbon nanocapsules by arc discharge. J. Cryst. Growth 1997, 172, 163–170.
Lijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58.
Yang, T. T.; Jia, P.; Liu, Q. N.; Zhang, L. Q.; Du, C. C.; Chen, J. Z.; Ye, H. J.; Li, X. M.; Li, Y. S.; Shen, T. D. et al. Air-stable lithium spheres produced by electrochemical plating. Angew. Chem. 2018, 130, 12932–12935.
Zhang, L. Q.; Tang, Y. S.; Liu, Q. N.; Yang, T. T.; Du, C. C.; Jia, P.; Wang, Z. F.; Tang, Y. F.; Li, Y. F.; Shen, T. D. et al. Probing the charging and discharging behavior of K-CO2 nanobatteries in an aberration corrected environmental transmission electron microscope. Nano Energy 2018, 53, 544–549.
Liu, Q. N.; Yang, T. T.; Du, C. C.; Tang, Y. F.; Sun, Y.; Jia, P.; Chen, J. Z.; Ye, H. J.; Shen, T. D.; Peng, Q. M. et al. In situ imaging the oxygen reduction reactions of solid state Na-O2 batteries with CuO nanowires as the air cathode. Nano Lett. 2018, 18, 3723–3730.
Liu, Q. N.; Geng, L.; Yang, T. T.; Tang, Y. F.; Jia, P.; Li, Y. S.; Li, H.; Shen, T. D.; Zhang, L. Q.; Huang, J. Y. In-situ imaging electrocatalysis in a Na-O2 battery with Au-coated MnO2 nanowires air cathode. Energy Storage Mater. 2019, 19, 48–55.
Huang, J. Y.; Zhong, L.; Wang, C. M.; Sullivan, J. P.; Xu, W.; Zhang, L. Q.; Mao, S. X.; Hudak, N. S.; Liu, X. H.; Subramanian, A. et al. In situ observation of the electrochemical lithiation of a single SnO2 nanowire electrode. Science 2010, 330, 1515–1520.
Lee, C.; Yang, W. T.; Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789.
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652.
Francl, M. M.; Pietro, W. J.; Hehre, W. J.; Binkley, J. S.; Gordon, M. S.; Defrees, D. J.; Pople, J. A. Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J. Chem. Phys. 1982, 77, 3654–3665.
Blaudeau, J. P.; McGrath, M. P.; Curtiss, L. A.; Radom, L. Extension of Gaussian-2 (G2) theory to molecules containing third-row atoms K and Ca. J. Chem. Phys. 1997, 107, 5016–5021.
Wang, Z. F.; Tang, Y. F.; Zhang, L. Q.; Li, M.; Shan, Z. W.; Huang, J. Y. In situ TEM observations of discharging/charging of solid-state lithium-sulfur batteries at high temperatures. Small 2020, 16, 2001899.
Zhang, D. W.; Zhao, Y. B.; Goodenough, J. B.; Lu, Y. H.; Chen, C. H.; Wang, L.; Liu, J. W. Exfoliation from carbon nanotubes versus tube size on lithium insertion. Electrochem. Commun. 2011, 13, 125–128.
Wu, S. T.; Wu, H. Q.; Zou, M. C.; Shi, X. W.; Yuan, Y. J.; Bai, W. F.; Cao A. Y. Short-range ordered graphitized-carbon nanotubes with large cavity as high-performance lithium-ion battery anodes. Carbon 2020, 158, 642–650.
Zhao, J.; Gao, Q. Y.; Gu, C.; Yang, Y. Preparation of multi-walled carbon nanotube array electrodes and its electrochemical intercalation behavior of Li ions. Chem. Phys. Lett. 2002, 358, 77–82.
Jia, P.; Yang, T. T.; Liu, Q. N.; Yan, J. T.; Shen, T. D.; Zhang, L. Q.; Liu, Y. N.; Zhao, X. X.; Gao, Z. Y.; Wang, J. et al. In-situ imaging Co3O4 catalyzed oxygen reduction and evolution reactions in a solid state Na-O2 battery. Nano Energy 2020, 77, 105289.
Huo, H. Y.; Luo, J.; Thangadurai, V.; Guo, X. X.; Nan, C. W.; Sun, X. L. Li2CO3: A critical issue for develo** solid garnet batteries. ACS Energy Lett. 2020, 5, 252–262.
Rao, R. P.; Gu, W. Y.; Sharma, N.; Peterson, V. K.; Avdeev, M.; Adams, S. In situ neutron diffraction monitoring of Li7La3Zr2O12 formation: Toward a rational synthesis of garnet solid electrolytes. Chem. Mater. 2015, 27, 2903–2910.
Di, Y. Z.; Feng, N. X.; Dong, W. W.; Peng, J. P.; Wang, Y. W. Study on thermal decomposition of Li2CO3 in production of lithium by vacuum thermal reduction process. Nonf. Met. (Extract. Metall.) 2019, 28–30.
Liu, X. H.; Huang, S.; Picraux, S. T.; Li, J.; Zhu, T.; Huang, J. Y. Reversible nanopore formation in Ge nanowires during lithiation-delithiation cycling: An in situ transmission electron microscopy study. Nano Lett. 2011, 11, 3991–3997.
Li, X. L.; Zhou, J. W.; Zhang, J. X.; Li, M.; Bi, X. X.; Liu, T. C.; He, T.; Cheng, J. L.; Zhang, F.; Li, Y. P. et al. Bamboo-Like nitrogen-doped carbon nanotube forests as durable metal-free catalysts for self-powered flexible Li-CO2 batteries. Adv. Mater., 2019, 31, 1903852.
Lu, Y.; Cai, Y. C.; Zhang, Q.; Ni, Y. X.; Zhang, K.; Chen, J. Rechargeable K-CO2 batteries with a KSn anode and a carboxylcontaining carbon nanotube cathode catalyst. Angew. Chem. 2021, 133, 9626–9631.
Rassolov, V. A.; Pople, J. A.; Ratner, M. A.; Windus, T. L. 6-31G* basis set for atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229.
Rassolov, V. A.; Ratner, M. A.; Pople, J. A.; Redfern, P. C.; Curtiss, L. A. 6-31G* basis set for third-row atoms. J. Comput. Chem. Commun. 2001, 22, 976–984.
Abraham, K. M. Prospects and limits of energy storage in batteries. J. Phys. Chem. Lett. 2015, 6, 830–844.
Li, Y. T.; Chen, X.; Dolocan, A.; Cui, Z. M.; **n, S.; Xue, L. G.; Xu, H. H.; Park, K.; Goodenough, J. B. Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J. Am. Chem. Soc. 2018, 140, 6448–6455.
Dissanayake, M. A. K. L.; Mellander, B. E. Phase diagram and electrical conductivity of the Li2SO4-Li2CO3 system. Solid State Ionics 1986, 21, 279–285.
Acknowldgements
This work was financially supported by the the National Natural Science Foundation of China (Nos. 52022088, 51971245, 51772262, 21406191, U20A20336, and 21935009), Bei**g Natural Science Foundation (No. 2202046), Selective funding for provincial postdoctoral research projects (No. B2019003018), Fok Ying-Tong Education Foundation of China (No. 171064), Natural Science Foundation of Hebei Province (Nos. B2020203037, and B2018203297), and Hunan Innovation Team (No. 2018RS3091).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2021_3514_MOESM4_ESM.pdf
In-situ imaging the electrochemical reactions of Li-CO2 nanobatteries at high temperatures in an aberration corrected environmental transmission electron microscope
Rights and permissions
About this article
Cite this article
Jia, P., Yu, M., Zhang, X. et al. In-situ imaging the electrochemical reactions of Li-CO2 nanobatteries at high temperatures in an aberration corrected environmental transmission electron microscope. Nano Res. 15, 542–550 (2022). https://doi.org/10.1007/s12274-021-3514-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3514-9