Abstract
Objective
Many studies have reported that animals will display collision avoidance behavior when the size of retinal image of an object reaches a threshold. The present study aimed to investigate the neural correlates underlying the frog collision avoidance behavior.
Methods
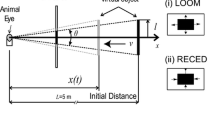
Different types of visual stimuli simulating the retinal image of an approaching or a recessing object were generated by a computer and presented to the right eye of frog. A multielectrode array was used to examine the activity of collision-sensitive neurons, and single electrode recordings were employed to quantify visual parameter (s) of the frog collision-sensitive neurons.
Results
The multielectrode array revealed that 40 neurons in the optic tectum showed selective responsiveness to objects approaching on a direct collision course. The response profiles of these collision-sensitive neurons were similar to those of lobula giantmovement detector (LGMD) in the locust or to those of η neurons in the pigeon. However, the receptive field (RF) size of the frog neurons [(18.5±3.8) °, n=33)] was smaller than those of collision-sensitive neurons of the locust and the pigeon. Multielectrode recordings also showed that the collision-sensitive neurons were activated only when the focus of expansion of a looming retinal image was located within the center of its RF. There was a linear relationship between the parameter l/v (l denotes half-size of the object, v denotes approaching velocity) and time-to-collision (time difference between the peak of the neuronal activity and the predictive collision) in 16 collisionsensitive neurons. Theoretical consideration showed that the peak firing rate always occurred at a fixed delay of (60.1 ± 39.5) ms (n=16) after the object had reached a constant angular size of (14.8 ± 3.4)° (n=16) on the retina.
Conclusion
The results may help clarify the mechanisms underlying the collision avoidance behavior in bullfrog.
摘要
目的
许多研究显示, 当一个靠**的物体在视网膜的成像达到一定阈值时, 动物即表现出逃避行为。 本研究旨在探讨青蛙的这种复杂的神经活动机理。
方法
用计算机模拟不同类型的视觉刺激, 并用多电极和单电极记录方法来记录碰撞刺激引起的视顶盖细胞的神经活动。
结果
多电极记录显示视顶盖神经细胞对碰撞刺激的反应具有选择性, 且反应特性相似于蝗虫的小叶大运动侦察器神经元(lobula giant-movement detector, LGMD)和鸽子的η碰撞神经细胞。 但与这些细胞相比, 视顶盖神经细胞受容野的大小相对较小。 单电极记录研究了这些碰撞敏感细胞的视觉参数。 其视觉参数l/v 和即将碰撞时间(time-to-collision)之间存在线性关系。
结论
这些结果进一步证实了青蛙视顶盖的碰撞敏感细胞的神经活性与一定的视网膜成像大小有关, 有助于进一步阐明青蛙逃避行为的内在机制。
Similar content being viewed by others
References
Ball W, Tronick E. Infant responses to impending collision: Optical and real. Science 1971, 171: 818–820.
Hayes WN, Saiff EI. Visual alarm reactions in turtles. Anim Behav 1967, 15: 102–106.
Schiff W, Caviness JA, Gibson JJ. Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science 1962, 136: 982–983.
Lee DN, Reddish PE. Plummeting gannets: A paradigm of ecological optics. Nature 1981, 293: 293–294.
Schiff W. Perception of impending collision: A study of visually directed avoidant behavior. Psychol Monogr 1965, 79: 1–26.
Wagner H. Flow-field variables trigger landing in flies. Nature 1982, 297: 147–148.
Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci 1999, 19: 1122–1141.
Gabbiani F, Mo C, Laurent G. Invariance of angular threshold computation in a wide-field looming-sensitive neuron. J Neurosci 2001, 21: 314–329.
Hatsopoulos N, Gabbiani F, Laurent G. Elementary computation of object approach by a wide-field visual neuron. Science 1995, 270: 1000–1003.
Rind FC, Simmons PJ. Orthopteran DCMD neuron: A reevaluation of responses to moving objects. I. Selective responses to approaching objects. J Neurophysiol 1992, 68: 1654–1666.
Rind FC, Simmons PJ. Seeing what is coming: building collision-sensitive neurones. TINS 1999, 22(5): 215–220.
Schlotterer GR. Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Can J Zool 1977, 55: 1372–1376.
Sun H, Frost BJ. Computation of different optical variables of looming objects in pigeon nucleus rotundus neurons. Nature Neurosci 1998, 1: 296–303.
Wu LQ, Niu YQ, Yang J, Wang SR. Tectal neurons signal impending collision of looming objects in the pigeon. Eur J Neurosci 2005, 22(9): 2325–2331.
**ao Q, Li DP, Wang SR. Looming-sensitive responses and receptive field organization of telencephalic neurons in the pigeon. Brain Res Bull 2006, 68: 322–328.
Holmqvist MH, Srinivasan MV. A visually evoked escape response of the housefly. J Comp Physiol A 1991, 169: 451–459.
Regan D, Cynader M. Neurons in area 18 of cat visual cortex selectively sensitive to changing size: Nonlinear interactions between responses to two edges. Vision Res 1979, 19: 699–711.
Wicklein M, Strausfeld NJ. Organization and significance of neurons that detect change of visual depth in the hawk moth Manduca sexta. J Comp Neurol 2000, 424: 356–376.
Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature 2002, 420: 320–324.
Gabbiani F, Krapp HG, Hatsopoulos N, Mo CH, Koch C, Laurent G. Multiplication and stimulus invariance in a looming-sensitive neuron. J Physiol Paris 2004, 98: 19–34.
Gray JR, Lee JK, Robertson RM. Activity of descending contralateral movement detector neurons and collision avoidance behaviour in response to head-on visual stimuli in locusts. J Comp Physiol A 2001, 187: 115–129.
Matheson T, Rogers SM, Krapp HG. Plasticity in the visual system is correlated with a change in lifestyle of solitarious and gregarious locusts. J Neurophysiol 2004, 91(1): 1–12.
Santer RD, Simmons PJ, Rind FC. Gliding behaviour elicited by lateral looming stimuli in flying locusts. J Comp Physiol A 2005, 191: 61–73.
Lee DN, Davies MNO, Green PR, Weel FRVD. Visual control of velocity of approach by pigeons when landing. J Exp Biol 1993, 180: 85–104.
Wang Y, Frost BJ. Time to collision is signaled by neurons in the nucleus rotundus of pigeons. Nature 1992, 356: 236–238.
Yamamoto K, Nakata M, Nakagawa H. Input and output characteristics of collision avoidance behavior in the frog Rana catesbeiana. Brain Behav Evol 2003, 62: 201–211.
Ewert JP. Single unit response of the toad’s (Bufo americanus) caudal thalamus to visual objects. Z Vergl Physiol 1971, 74: 81–102.
Grüsser OJ, Grüsser-Cornehls U. Neurophysiology of the anuran visual system. In: Frog neurobiology, edited by Llinás R and Precht W. Berlin, Heidelberg, New York: Springer Verlag, 1976: 297–385.
Kang H, Nakagawa H. Collision-sensitive neurons in the optic tectum of the bullfrog (Rana catesbeiana). Int Congress Series 2006, 1291: 145–148.
Rowell CHF, O’shea M, Williams JLD. The neuronal basis of a sensory analyzer, the acridic movement detector system. J Exp Biol 1977, 68: 157–185.
Bower TGR, Broughton JM, Moore MK. Infant responses to approaching objects: an indicator of response to distal variables. Percept Psychophys 1970, 9: 193–196.
Lee DN. A theory of visual control of braking based on information about time-to-collision. Perception 1976, 5: 437–459.
Lee DN, Young DS. Visual timing of interceptive action. In: Brain mechanisms and spatial vision, edited by Ingle DJ, Jeannerod M, and Lee DN. Dordrecht: Martinus Nijhoff, 1985: 1–30.
Regan D, Hamstra SJ. Dissociation of discrimination thresholds for time to contact and for rate of angular expansion. Vision Res 1993, 4: 447–462.
Wunderlich G, Marshall JC, Amunts K, Weiss PH, Mohlberg H, Zafiris O, et al. The importance of seeing it coming: a functional magnetic resonance imaging study of motion-in-depth towards the human observer. Neuroscience 2002, 112(3): 535–540.
Wang Y, Jiang S, Frost BJ. Visual processing in pigeon nucleus rotundus: Luminance, color, motion, and looming subdivisions. Vis Neurosci 1993, 10: 21–30.
Gray JR. Habituated visual neurons in locusts remain sensitive to novel looming objects. J Exp Biol 2005, 208: 2515–2532.
Judge SJ, Rind FC. The locust DCMD, a movement-detecting neurone tightly tuned to collision trajectories. J Exp Biol 1997, 200: 2209–2216.
Rind FC, Simmons PJ. Signaling of object approach by the DCMD neuron of the locust. J Neurophysiol 1997, 77: 1029–1033.
Simmons PJ, Rind FC. Orthopteran DCMD neuron: A reevaluation of responses to moving objects. II. Critical cues for detecting approaching objects. J Neurophysiol 1992, 68: 1667–1682.
Burrows M, Rowell CHF. Connections between descending visual interneurons and metathoracic motoneurons in the locust. J Comp Physiol A 1973, 85: 221–234.
O’shea M, Rowell CHF, Williams JLD. The anatomy of a locust visual interneurone: the descending contralateral movement detector. J Exp Biol 1974, 60: 1–12.
Simmons PJ. Connexions between a movement-detecting visual interneurone and flight motoneurones of a locust. J Exp Biol 1980, 86: 87–97.
Nakagawa H, Yamamoto K. Input-ouput characteristics of collision avoidance behavior and path length-dependent tuning of putative collision-sensitive neurons of the frog Rana catesbeiana. Int Congress Series 2004, 1269: 65–68.
Gaze RM. The representation of the retina on the optic lobe of the frog. Q J Exp Physio l 1958, 43: 209–214.
Potter HD. Structural characteristics of cell and fiber populations in the optic tectum of the frog (Rana catesbeiana). J Comp Neurol 1969, 136: 203–232.
Székely G, Lázár G. Cellular and synaptic architecture of the optic tectum. In: Frog neurobiology, edited by Llinás R and Precht W. Berlin, Heidelberg, New York: Springer Verlag, 1976: 407–434.
Ingle DJ, Hoff K vS. Visually elicited evasive behavior in frogs. Bioscience 1990, 40: 284–291.
Ingle D. Two visual systems in the frog. Science 1973, 181: 1053–1055.
Ingle D. Detection of stationary objects by frogs following optic tectum ablation. J Comp Physiol Psychol 1977, 91: 1359–1364.
Waldeck RF, Gruberg ER. Studies on the optic chiasm of the leopard frog. I. Selective loss of visually elicited avoidance behavior after optic chiasm hemisection. Brain Behav Evol 1995, 46: 84–94.
King JG, Lettvin JY, Gruberg ER. Selective, unilateral, reversible loss of behavioral responses to looming stimuli after injection of tetrodotoxin or cadmium chloride into the frog optic nerve. Brain Res 1999, 841: 20–26.
Ishikane H, Gangi M, Honda S, Tachibana M. Synchronized retinal oscillations encode essential information for escape behavior in frogs. Nat Neurosci 2005, 8(8): 1087–1095.
Hartline HK. The response of single optic nerve fibers of the vertebrate eye to illumination of the retina. Am J Phyiol 1938, 121: 400–415.
Lettvin JY, Maturana HR, McCulloch WS, Pitts WH. What the frog’s eye tells the frog’s brain. Proc I R E 1959, 47: 1940–1951.
Gabbiani F, Cohen I, Laurent G. Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron. J Neurophysiol 2005, 94: 2150–2161.
O’shea M, Rowell CHF. Protection from habituation by lateral inhibition. Nature 1975, 254: 53–55.
Rind FC. Motion detectors in the locust visual system: from biology to robot sensors. Microsc Res Tech 2002, 56: 256–269.
Duffy CJ, Wurtz RH. Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J Neurophysiol 1991a, 65(6): 1329–1345.
Tohyama K, Fukushima K. Neural network model for extracting optic flow. Neural Netw 2005, 18: 549–556.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kang, HJ., Li, XH. Response properties and receptive field organization of collision-sensitive neurons in the optic tectum of bullfrog, Rana catesbeiana . Neurosci. Bull. 26, 304–316 (2010). https://doi.org/10.1007/s12264-010-0306-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-010-0306-8