Abstract
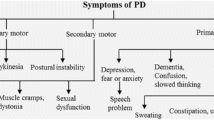
Parkinson’s disease (PD) is caused by progressive degeneration of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc), resulting in the deficiency of DA in the striatum. Thus, symptoms are developed, such as akinesia, rigidity and tremor. The aetiology of neuronal death in PD still remains unclear. Several possible mechanisms of the degeneration of dopaminergic neurons are still elusive. Various mechanisms of neuronal degeneration in PD have been proposed, including formation of free radicals, oxidative stress, mitochondrial dysfunction, excitotoxicity, calcium cytotoxicity, trophic factor deficiency, inflammatory processes, genetic factors, environmental factors, toxic action of nitric oxide, and apoptosis. All these factors interact with each other, inducing a vicious cycle of toxicity causing neuronal dysfunction, atrophy and finally cell death. Considerable evidence suggests that free radicals and oxidative stress may play key roles in the pathogenesis of PD. However, currently, drug therapy cannot completely cure the disease. DA replacement therapy with levodopa (L-Dopa), although still being a gold standard for symptomatic treatment of PD, only alleviates the clinical symptoms. Furthermore, patients usually experience severe side effects several years after the L-Dopa treatment. Until now, no therapy is available to stop or at least slow down the neurodegeneration in patients. Therefore, efforts are made not only to improve the effect of L-Dopa treatment for PD, but also to investigate new drugs with both antiparkinsonian and neuroprotective effects. Here, the advantages and limitations of current and future therapies for PD were dicussed. Current therapies include dopaminergic therapy, DA agonists, MAO-B inhibitor, COMT inhibitors, anticholinergic drugs, surgical procedures such as pallidotomy and more specifically deep brain stimulation of the globus pallidus pars interna (GPi) or subthalamic nucleus (STN), and stem cell transplantation.
摘要
帕金森氏病(Parkinson’s disease, PD)是由中脑黑质中多巴胺神经元变性, 导致纹状体系统多巴胺(DA)含量下降引起的神经病变。 其特征性症状包括震颤、 僵硬和运动徐缓等。 目前为止, 帕金森氏病神经元死亡的病因仍不清楚。 具体的神经变性机制包括自由基生成、 氧化应激、 线粒体异常、 兴奋性中毒、 钙中毒、 营养因子不足、 炎症过程、 一氧化氮毒性和细胞调亡。 这些因素相互增**形成恶性循环导致神经功能异常、 萎缩, 最终导致多巴胺神经元死亡。 大量实验提示在PD 病理过程中, 自由基的生成和氧化应激起关键作用。 目前, 药物疗法并不能治愈PD。 尽맜左旋多巴(L-Dopa)替代疗法一直是控制PD 症状的标准, 但其只能缓解临床症状, 并且L-Dopa 长期治疗会引起多种副作用。 目前尚无可行的疗法能遏制或减缓神经元变性。 因此, 研究不仅要致力于改善和延长L-Dopa对PD的治疗效果, 还要研发兼具抗PD与神经保护功能的药物。 本文综述了当前各种PD疗法的优缺点。 这些疗法包括DA 治疗、 DA 激动剂、 单胺氧化酶-B 抑制剂、 儿茶酚-O-甲基转移酶抑制剂、 抗谷氨酸药、 胆 碱能药物、 外科手术(深部大脑苍白球或丘脑术)和干细胞移植术等。 同时, 基于PD 病理过程, 对未来的药物神经保护作一展望。
Similar content being viewed by others
References
Pahwa R. Understanding Parkinson’s disease: an update on current diagnostic and treatment strategies. J Am Med Dir Assoc 2006, 7(S2): 4–10.
Chen SD. Treatment Guidebook of Parkinson’s disease in China. Chin J Neurol 2009, 42(5).
Odin P, Wolters E, Antonini A. Continuous dopaminergic stimulation achieved by duodenal levodopa infusion. Neurol Sci 2008, 29(S5): S387–388.
Cao XB, Guan Q, Xu Y, Wang L, Sun SG. Mechanism of overactivation in direct pathway mediated by dopamine D(1) receptor in rats with levodopa-induced dyskinesia. Neurosci Bull 2006, 22(3): 159–164.
Pahwa R, Lyons KE. Levodopa-related wearing-off in Parkinson’s disease: identification and management. Curr Med Res Opin 2009, 25(4): 841–849.
Nashatizadeh MM, Lyons KE, Pahwa R. A review of ropinirole prolonged release in Parkinson’s disease. Clin Interv Aging 2009, 4(1): 179–186.
Antonini A, Barone P. Dopamine agonist-based strategies in the treatment of Parkinson’s disease. Neurol Sci 2008, 29(S5): S371–374.
Binder S, Deuschl G, Volkmann J. Effect of cabergoline on parkinsonian tremor assessed by long-term actigraphy. Eur Neurol 2009, 61(3): 149–153.
Lew MF, Pahwa R, Leehey M, Bertoni J, Kricorian G, Zydis selegiline Study Group. Safety and efficacy of newly formulated selegiline orally disintegrating tablets as an adjunct to levodopa in the management of ‘off’ episodes in patients with Parkinson’s disease. Curr Med Res Opin 2007, 23(4): 741–750.
Uzun M, Alp R, Uzlu E, Alp S, Citil M, Topcu B, et al. Investigation of oral selegiline and rasagiline administration on QT interval in conscious rabbits. Eur Rev Med Pharmacol Sci 2009, 13(2): 95–98.
Weinreb O, Mandel S, Bar-Am O, Yogev-Falach M, Avramovich-Tirosh Y, Amit T, et al. Multifunctional neuroprotective derivatives of rasagiline as anti-Alzheimer’s disease drugs. Neurotherapeutics 2009, 6(1): 163–174.
Pellicano C, Benincasa D, Giovannelli M, Buttarelli FR, Ruggieri S, Pontieri FE. Entacapone in elderly Parkinsonian patients experiencing levodopa-related wearing-off: a pilot study. Neurol Res 2009, 31(1): 74–76.
Canesi M, Zecchinelli AL, Pezzoli G, Antonini A. Clinical experience of tolcapone in advanced Parkinson’s disease. Neurol Sci 2008, 29(S5): S380–382.
Alonso Navarro H, Sanz-Aiz A, Izquierdo L, Jimenez Jimenez FJ. Syndrome of inappropriate antidiuretic hormone secretion possibly associated with amantadine therapy in Parkinson disease. Clin Neuropharmacol 2009, 32(3): 167–168.
Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea 2008, 27(10): 1182–1185.
Lyons KE, Pahwa R. Deep brain stimulation and tremor. Neurotheapeutics 2008, 5(2): 331–338.
Tsai ST, Lin SH, Chou YC, Pan YH, Hung HY, Li CW, et al. Prognostic factors of subthalamic stimulation in Parkinson’s disease: A comparative study between short- and long-term effects. Stereotact Funct Neurosurg 2009, 87(4): 241–248.
Wijeyekoon R, Barker RA. Cell replacement therapy for Parkinson’s disease. Biochim Biophys Acta 2009, 1792(7): 688–702.
Lee PH, Park HJ. Bone marrow-derived mesenchymal stem cell therapy as a candidate disease-modifying strategy in Parkinson’s disease and multiple system atrophy. J Clin Neurol 2009, 5(1): 1–10.
Yuan H, Zheng JC, Liu P, Zhang SF, Xu JY, Bai LM. Pathogenesis of Parkinson disease: oxidative stress, environmental factors and inflammatory processes. Neurosci Bull 2007, 23(2): 125–130.
Schapira AH, Olanow CW. Rational for the use of dopamine agonists as neuroprotective agents in Parkinson’s disease. Ann Neurol 2003, 53(S3): S149–159.
Joyce JN, Woolsey C, Ryoo H, Borwege S, Hagner D. Low dose pramipexole is neuroprotective in the MPTP mouse model of Parkinson’s disease, and downregulates the dopamine transporter via the D3 receptor. BMC Biol 2004, 2(1): 22.
Wen HB, Zhang ZX, Luo Y. A randomized, double-blind and more center study on the curative effect & safety of Pramipexole treat Parkinson’s disease, compared with bromocriptine. Chin J Neurol 2006, 39: 604–608.
Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 2004, 61(7): 1044–1053.
Antonini A, Tolosa E. Apomorphine and levodopa infusion therapies for advanced Parkinson’s disease: selection criteria and patient management. Expert Rev Neurother 2009, 9(6): 859–867.
Yuan H, Liang LW, Chen ZJ, Ji HR, Wang MK, Zhang HY, et al. R-apomorphine protects against 6-hydroxydopamine-induced nigrostriatal damage in rat. Neurosci Bull 2006, 22(6): 331–338.
Kyriazis M. Neuroprotective, anti-apoptotic effects of apomophine. J Anti Aging Med 2003, 6(1): 21–28.
Stocchi F. Use of apomorphine in Parkinson’s disease. Neurol Sci 2008, 29(S5): S383–386.
Factor SA. Literature review: intermittent subcutaneous apomorphine therapy in Parkinson’s disease. Neurology 2004, 62(6 Suppl 4): S12–17.
Stacy M. Apomorphine: North American clinical experience. Neurology 2004, 62(6 Suppl 4): S18–21.
Ward RJ, Lallemand F, de Witte P, Dexter DT. Neurochemical pathways involved in the protective effects of nicotine and ethanol in preventing the development of Parkinson’s disease: potential targets for the development of new therapeutic agents. Prog Neurobiol 2008, 85(2): 135–147.
Perez XA, Oleary KT, Parameswaran N, Mcintosh JM, Quik M. Prominent role of alpha3/alpha6beta2* nAChRs in regulating evoked dopamine release in primate putamen: effect of long-term nicotine treatment. Mol Pharmacol 2009, 75(4): 938–946.
Yanagida T, Takeuchi H, Kitamura Y, Takata K, Minamino H, Shibaike T, et al. Synergistic effect of galantamine on nicotineinduced neuroprotection in hemiparkinsonian rat model. Neurosci Res 2008, 62(4): 254–261.
Hong DP, Fink AL, Uversky VN. Smoking and Parkinson’s disease: does nicotine affect alpha-synuclein fibrillation? Biochim Biophys Acta 2009, 1794(2): 282–290.
Dhanasekaran M, Karuppagounder SS, Uthayathas S, Wold LE, Parameshwaran K, Jayachandra Babu R, et al. Effect of dopaminergic neurotoxin MPTP/MPP+ on coenzyme Q content. Life Sci 2008, 83(3–4): 92–95.
Abdin AA, Hamouda HE. Mechanism of the neuroprotective role of coenzyme Q10 with or without L-dopa in rotenoneinduced parkinsonism. Neuropharmacology 2008, 55(8): 1340–1346.
Beal MF. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol 2003, 53(S3): S39–48.
Bensimon G, Ludolph A, Agid Y, Vidaihet M, Payan C, Leigh PN, et al. Riluzole treatment, survival and diagnostic criteria in Parkinson plus disorders: the NNIPPS study. Brain 2009, 132(Pt 1): 156–171.
Braz CA, Borges V, Ferraz HB. Effect of riluzole on dyskinesia and duration of the on state in Parkinson disease patients: a double-blind, placebo-controlled pilot study. Clin Neuropharmacol 2004, 27(1): 25–29.
Clarke CE, Cooper JA, Holdich TA. A randomized, double-blind, placebo-controlled, ascending-dose tolerability and safety study of remacemide as adjuvant therapy in Parkinson’s disease with response fluctuations. Clin Neuropharmacol 2001, 24(3): 133–138.
Blanchet PT, Metman LV, Chase TN. Renaissance of amantadine in treatment of Parkinson’s disease. Adv Neurol 2003, 91: 251–257.
Levin OS, Batukaeva LA. Efficacy of memantine in Parkinson’s disease with dementia. Zh Nevrol Psikhiatr Im S S Korsakova 2008, 108(12): 16–23.
Seeman P, Caruso C, Lasaga M. Memantine agonist action at dopamine D2High receptors. Synapse 2008, 62(2): 149–153.
Murray TK, Messenger MJ, Ward MA, Woodhouse S, Osborne DJ, Duty S, et al. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson’s disease. Pharmacol Biochem Behav 2002, 73(2): 455–466.
Eberling JL, Kells AP, Pivirotto P, Beyer J, Bringas J, Federoff HJ, et al. Functional effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in Parkinsonian Rhesus monkeys. Hum Gene Ther 2009, 20(5): 511–518.
Hurelbrink CB, Barker RA. The potential of GDNF as a treatment for Parkinson’s disease. Exp Neurol 2004, 185(1): 1–6.
Herzog CD, Brown L, Gammon D, Kruegel B, Lin R, Wilson A, et al. Expression, bioactivity, and safety 1 year after adeno-associated viral vector type 2-mediated delivery of neurturin to the monkey nigrostriatal system support cere-120 for Parkinson’s disease. Neurosurgery 2009, 64(4): 602–612; discussion 612–613.
Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 2009, 8(4): 382–397.
Bartels AL, Willemsen AT, Doorduin J, de Vries EF, Dierckx RA, Leenders KL. [(11)C]-PK11195 PET: Quantification of neuroinflammation and a monitor of anti-inflammatory treatment in Parkinson’s disease? Parkinsonism Relat Disord 2009, doi:10.1016/j.parkreldis.2009.05.005 [Epub ahead of print].
Zhu C, Wang X, Qiu L, Peeters-Scholte C, Hagberg H, Blomgren K. Nitrosylation precedes caspase-3 activation and translocation of apoptosis-inducing factor in neonatal rat cerebral hypoxia-ischaemia. J Neurochem 2004, 90(2): 462–471.
Silverman RB. Design of selective neuronal nitric oxide synthase inhibitors for the prevention and treatment of neurodegenerative diseases. Acc Chem Res 2009, 42(3): 439–451.
Rife T, Rasoul B, Pullen N, Mitchell D, Grathwol K, Kurth J. The effect of a promoter polymorphism on the transcription of nitric oxide synthase 1 and its relevance to Parkinson’s disease. J Neurosci Res 2009, 87(10): 2319–2325.
Padovan-Neto FE, Echeverry MB, Tumas V, Del-Bel EA. Nitric oxide synthase inhibition attenuates L-DOPA-induced dyskinesias in a rodent model of Parkinson’s disease. Neuroscience 2009, 159(3): 927–935.
Volpini R, Dal Ben D, Lambertucci C, Marucci G, Mishra RC, Ramadori AT, et al. Adenosine A2A receptor antagonists: new 8-substituted 9-ethyladenines as tools for in vivo rat models of Parkinson’s disease. ChemMedChem 2009, 4(6): 1010–1019.
Marucci G, Finaurini S, Buccioni M, Lammi C, Kandhavelu M, Volpini R, et al. In vitro metabolism studies of new adenosine A 2A receptor antagonists. Drug Metab Lett 2008, 2(4): 301–307.
Wang S, Jiang H, Qu L. Study on the mechanism of electroacupuncture scalp point penetration therapy in action on apoptosis in the Parkinson’s disease rat model. Chinese Acupuncture & Moxibustion 2009, 29(4): 309–313.
Zheng R, Zhou HY, Chen SD. The role of synphilin-1 in the pathogenesis of Parkinson’s disease. Neurosci Bull 2006, 22(4): 239–243.
Vekrellis K, **louri M, Emmanouilidou E, Stefanis L. Inducible over-expression of wild type alpha-synuclein in human neuronal cells leads to caspase-dependent non-apoptotic death. J Neurochem 2009, 109(5): 1348–1362.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, H., Zhang, ZW., Liang, LW. et al. Treatment strategies for Parkinson’s disease. Neurosci. Bull. 26, 66–76 (2010). https://doi.org/10.1007/s12264-010-0302-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-010-0302-z