Abstract
Objective
The neuroprotective roles of cyclooxygenase (COX) and lipooxygenase (LOX) inhibitors have been well documented. Quinolinic acid (QA) is a well-known excitotoxic agent that could induce behavioral, morphological and biochemical alterations similar with symptoms of Huntington’s disease (HD), by stimulating NMDA receptors. However, the exact roles of COX and LOX inhibitors in HD have not yet been explained. The present study aims to elucidate the effects of caffeic acid (a specific inhibitor for LOX), rofecoxib (a specific inhibitor for COX-2), and their combination in ameliorating QAinduced neurotoxicity in rats.
Methods
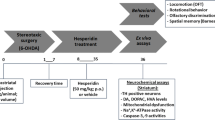
QA was injected into the right striatum of rats to induce neurotoxicity. Caffeic acid and rofecoxib were then orally administered separately. In the combination study, caffeic acid and rofecoxib were administered together. After that, a series of behavioral assessments were conducted to determine the effects of caffeic acid and rofecoxib, respectively, and the co-effect of caffeic acid and rofecoxib, against QA-induced neurotoxicity.
Results
Intrastriatal QA administration (300 nmol) not only induced a significant reduction in body weight and motor incoordination, but also altered the redox status (decreased glutathione and increased oxidized glutathione level) in striatum, as compared to the sham group. Moreover, chronic treatment with caffeic acid (5 mg/kg and 10 mg/kg, respectively, p.o.) or rofecoxib (10 mg/kg, p.o.) could significantly attenuate QA-induced behavioral alterations and restore the redox status in striatum. However, at the dose of 2.5 mg/kg, caffeic acid did not show any significant effects on these parameters in QA-treated rats. Furthermore, the combination of rofecoxib (10 mg/kg) and caffeic acid (5 mg/kg) could significantly protect against QA neurotoxicity.
Conclusion
The in vivo study indicates that excitotoxic injury to the brain might affect oxidant/antioxidant equilibrium by eliciting changes in glutathione. Moreover, the LOX and the COX pathways may be both involved in quinolinic-induced neurotoxicity, which provides a promising target for HD treatment.
摘要
目的
环氧和酶(COX)抑制剂和脂肪氧化酶(LOX)抑制剂已被证实具有神经保护作用, 但对其具体机制目前研究甚少。 喹啉酸具有兴奋毒性作用, 通过激活NMDA 受体, 引起类似于亨廷顿舞蹈症 (Huntington’s Disease, HD) 的症状, 包括行为、 形态以及生化水**上的各种异常。 本研究旨在探讨咖啡酸(LOX特异性抑制剂)和罗非考昔(COX特异性抑制剂)各自以及两者连用对喹啉酸引起的大鼠神经毒性的改善和修复作用。
方法
在大鼠右侧纹状体内注射喹啉酸, 诱导神经毒性。 随后每天给大鼠口服咖啡酸或罗非考昔, 或两者同时服用。 用一系列行为学及生化检测方法检测咖啡酸和罗非考昔, 以及两者连用对喹啉酸诱导的大鼠行为变化及谷胱甘肽氧化还原紊乱的改善和修复作用。
结果
在纹状体注射喹啉酸不仅能降低大鼠体重, 引起运动失调, 而且能破坏纹状体内氧化还原间的**衡, 表现为谷胱甘肽水**降低, 以及氧化谷胱甘肽水**升高。 长期服用咖啡酸或罗非考昔, 以及两者连用都能显著减轻喹啉酸引起的行为变化, 修复氧化还原水**的**衡。 而当剂量为2.5 mg/kg 时, 咖啡酸未表现出任何保护作用。
结论
本实验结果表明, 大脑的兴奋性中毒有可能通过改变谷胱甘肽的水**影响氧化与抗氧化间的**衡。 环氧合酶和脂肪氧化酶通路都可能参与了喹啉酸诱导的神经毒性过程。 这些结果为治疗HD提供了研究靶点。
Similar content being viewed by others
References
Beal MF. Neurochemistry and toxin models in Huntington’s disease. Curr Opin Neurol 1994, 7(6): 542–547.
Sanberg PR, Calderon SF, Giordano M, Tew JM, Norman AB. The quinolinic acid model of Huntington’s disease: locomotor abnormalities. Exp Neurol 1989, 105: 45–53.
Furtado JC, Mazurek MF. Behavioral characterization of quinolinate-induced lesions of the medial striatum: relevance for Huntington’s disease. Exp Neurol 1996, 138(1): 158–168.
Roberts RC, Ahn A, Swartz KJ, Beal MF, DiFiglia M. Intrastriatal injections of quinolinic acid or kainic acid: differential patterns of cell survival and the effects of data analysis on outcome. Exp Neurol 1993, 124(2): 274–282.
Norman AB, Giordano M, Sandberg PR. Fetal striatal tissue graft into excitotoxin-lesioned striatum: pharmacological and behavioral aspects. Pharmacol Biochem Behav 1989, 34: 139–147.
Reynolds NC, Lin W, Cameron CM, Roerig DL. Differential response of extracellular GABA to intrastriatal perfusions of 3-nitropropionic acid and quinolinic acid in the rat. Brain Res 1997, 778: 140–149.
Choi DW. Excitotoxic cell death. J Neurobiol 1992, 23(9): 1261–1276.
Hurley SD, Olschowka JA, O’Banion MK. Cyclooxygenase inhibition as a strategy to ameliorate brain injury. J Neurotrauma 2002, 19(1): 1–15.
Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res 1986, 59(6): 612–619.
Benzi G, Moretti A. Age- and peroxidative stress-related modifi cations of the cerebral enzymatic activities linked to mitochondria and the glutathione system. Free Radic Biol Med 1995, 19(1): 77–101.
Cooper JL, Meister A. 1992. Glutathione in the brain: disorders of glutathione metabolism. In: Rosenberg, A. (Ed.), The Molecular and Genetic Basis of Neurological Disorders. Ruttle Graphics, New York, pp. 209–238.
Gerlach M, Riederer P, Youdim MBH. 1996. Molecular mechanisms for neurodegeneration. Synergism between reactive oxygen species, calcium, and excitotoxic amino acids. In: Battistin, L., Scarlato, G., Caraceni, T., Ruggieri, S. (Eds.), Parkinsons Disease, vol. 69. Lippincott-Raven Publishers, Philadelphia, pp. 177–194.
Krieglstein J. Excitotoxicity and neuroprotection. Eur J Pharm Sci 1997, 5: 181–187.
Froissard P, Monrocq H, Duval D. Role of glutathione metabolism in the glutamate-induced programmed cell death of neuronal-like PC12 cells. Eur J Pharmacol 1997, 326: 93–99.
Kart A, Cigremis Y, Karaman M, Ozen H. Caffeic acid phenethyl ester (CAPE) ameliorates cisplatin-induced hepatotoxicity in rabbit. Exp Toxicol Pathol 2009, 4 [Epub ahead of print].
Sul D, Kim HS, Lee D, Joo SS, Hwang KW, Park SY. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci 2009, 84(9–10): 257–262.
Rahamathulla M, Hv G, Rathod N. Solubility and dissolution improvement of Rofecoxib using solid dispersion technique. Pak J Pharm Sci 2008, 21(4): 350–355.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Academic Press, San Diego; 2007, 6th edition: 256.
Kumar P, Padi SS, Naidu PS, Kumar A. Cyclooxygenase inhibition attenuates 3-nitropropionic acid-induced neurotoxicity in rats: possible antioxidant mechanisms. Fundam Clin Pharmacol 2007, 21(3): 297–306.
Kulkarni SK. Hand book of experimental pharmacology (3rd edition). Delhi, Vallabh Prakashan, 1999.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959, 82: 70–77.
Zahler WL, Cleland WW. A specific and sensitive assay for disulfides. J Biol Chem 1968, 243: 716–719.
Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem 1949, 177(2): 751–766.
Moyanova SG, Kortenska LV, Mitreva RG, Pashova VD, Ngomba RT, Nicoletti F. Multimodal assessment of neuroprotection applied to the use of MK-801 in the endothelin-1 model of transient focal brain ischemia. Brain Res 2007, 11(1153): 58–67.
Estrada Sánchez AM, Mejía-Toiber J, Massieu L. Excitotoxic neuronal death and the pathogenesis of Huntington’s disease. Arch Med Res 2008, 39(3): 265–276.
Obrenovitch TP. Quinolinic acid accumulation during neuroinflammation. Does it imply excitotoxicity? Ann N Y Acad Sci 2001, 939: 1–10.
Datta K, Biswal SS, Kehrer JP. The 5-lipoxygenase-activating protein (FLAP) inhibitor, MK886, induces apoptosis independently of FLAP. Biochem J 1999, 1(340) (Pt 2): 371–375.
Klegeris A, McGeer PL. Cyclooxygenase and 5-lipoxygenase inhibitors protect against mononuclear phagocyte neurotoxicity. Neurobiol Aging 2002, 23(5): 787–794.
Bishnoi M, Patil CS, Kumar A, Kulkarni SK. Co-administration of acetyl-11-keto-beta-boswellic acid, a specific 5-lipoxygenase inhibitor, potentiates the protective effect of COX-2 inhibitors in kainic acid-induced neurotoxicity in mice. Pharmacology 2007, 79(1): 34–41.
Wise-Faberowski L, Pearlstein RD, Warner DS. NMDA-induced apoptosis in mixed neuronal/glial cortical cell cultures: the effects of isoflurane and dizocilpine. J Neurosurg Anesthesiol 2006, 18(4): 240–246.
Beal MF, Ferrante RJ, Swartz KJ, Kowall NW. Chronic quinolinic acid lesions in rats closely resemble Huntington’s disease. J Neurosci 1991, 11(6): 1649–1659.
Vazey EM, Chen K, Hughes SM, Connor B. Transplanted adult neural progenitor cells survive, differentiate and reduce motor function impairment in a rodent model of Huntington’s disease. Exp Neurol 2006, 199(2): 384–396.
Borlongan CV, Randall TS, Cahill DW, Sanberg PR. Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res 1995, 676: 231–234.
Picconi B, Passino E, Sgobio C, Bonsi P, Barone I, Ghiglieri V, et al. Plastic and behavioral abnormalities in experimental Huntington’s disease: a crucial role for cholinergic interneurons. Neurobiol Dis 2006, 22(1): 143–152.
Scattoni ML, Valanzano A, Pezzola A, March ZD, Fusco FR, Popoli P, et al. Adenosine A2A receptor blockade before striatal excitotoxic lesions prevents long term behavioural disturbances in the quinolinic rat model of Huntington’s disease. Behav Brain Res 2007, 176(2): 216–221.
Bordelon YM, Chesselet MF, Ereciñska M, Silver IA. Effects of intrastriatal injection of quinolinic acid on electrical activity and extracellular ion concentrations in rat striatum in vivo. Neuroscience 1998, 83(2): 459–469.
Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinás R, et al. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A 2005, 102(7): 2602–2607.
Soliman D, Hamming KS, Matemisz LC, Light PE. Reactive oxygen species directly modify sodium-calcium exchanger activity in a splice variant-dependent manner. J Mol Cell Cardiol. 2009, May 27 [Epub ahead of print].
Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta 2009, Mar 17 [Epub ahead of print]
Stanika RI, Pivovarova NB, Brantner CA, Watts CA, Winters CA, Andrews SB. Coupling diverse routes of calcium entry to mitochondrial dysfunction and glutamate excitotoxicity. Proc Natl Acad Sci U S A 2009, May 29 [Epub ahead of print]
Hansson O, Guatteo E, Mercuri NB, Bernardi G, Li XJ, Castilho RF, et al. Resistance to NMDA toxicity correlates with appearance of nuclear inclusions, behavioural deficits and changes in calcium homeostasis in mice transgenic for exon 1 of the huntington gene. Eur J Neurosci 2001, 14(9): 1492–1504.
Kelley KA, Ho L, Winger D, Freire-Moar J, Borelli CB, Aisen PS, et al. Potentiation of excitotoxicity in transgenic mice overexpressing neuronal cyclooxygenase-2. Am J Pathol 1999, 155(3): 995–1004.
Manev H, Uz T, Sugaya K, Qu T. Putative role of neuronal 5-lipoxygenase in an aging brain. FASEB J 2000, 14(10): 1464–1469.
Misko TP, Highkin MK, Veenhuizen AW, Manning PT, Stern MK, Currie MG, et al. Characterization of the cytoprotective action of peroxynitrite decomposition catalysts. J Biol Chem 1998, 273(25): 15646–15653.
Virág L, Szabó E, Gergely P, Szabó C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett 2003, 11(140–141): 113–124.
Szabó C. Multiple pathways of peroxynitrite cytotoxicity. Toxicol Lett 2003, 11(140–141): 105–112.
Reed DJ. Regulation of reductive process by glutathione. Biol Chem Pharmacol 1986, 35: 7–13.
Aries I, Jakoby WB. Glutathione metabolism and functions. Kroc Found Ser C 1976: 1-382.
Reed DJ, Fariss MW. Glutathione depletion and susceptibility. Pharmacol Rev 1984, 36: 525–533
Munday R, Winterbourn CC. Reduced glutathione in combination with superoxide dismutase as an important biological antioxidant defence mechanism. Biochem Pharmacol 1989, 38: 4349–4352.
Thifault C, Aumont N, Quirion R, Poirier J. Effect of MPTP and L-deprenyl on antioxidant enzymes and lipid peroxidation levels in mouse brain. J Neurochem 1995, 65: 2725–2733.
Barker JE, Heales SJR, Cassidy A, BolanÄ os JP, Land JM, Clark JB. Depletion of brain glutathione results in a decrease of glutathione reductase, an enzyme susceptible to oxidative damage. Brain Res 1996, 716: 118–122.
Babu GN, Bawari M. Single microinjection of L-glutamate induces oxidative stress in discrete regions of rat brain. Biochem Mol Biol Int 1997, 43: 1207–1217.
Murphy TH, Schnaar RL, Coyle JT. Immature cortical neurons are uniquely sensitive to glutamate toxicity by inhibition of cysteine uptake. FASEB J 1990, 4: 1624–1633.
Olanow CW. A radical hypothesis for neurodegeneration. Trends Neurosci 1993, 16: 39–44.
Pereira CMF, Oliveira CR. Glutamate toxicity on a PC12 cell line involves glutathione (GSH) depletion and oxidative stress. Free Radic Biol Med 1997, 23: 637–647.
Kumar A, Seghal N, Padi SV, Naidu PS. Differential effects of cyclooxygenase inhibitors on intracerebroventricular colchicineinduced dysfunction and oxidative stress in rats. Eur J Pharmacol 2006, 551: 58–66.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalonia, H., Kumar, P., Kumar, A. et al. Effects of caffeic acid, rofecoxib, and their combination against quinolinic acid-induced behavioral alterations and disruption in glutathione redox status. Neurosci. Bull. 25, 343–352 (2009). https://doi.org/10.1007/s12264-009-0513-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12264-009-0513-3