Abstract
Purpose
This study aims to formulate an ophthalmic gel-free reservoir (GFR) system for once-a-day (OD) delivery of timolol maleate for the treatment of glaucoma.
Methods
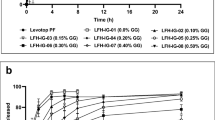
The GFR system contains hydroxypropyl methylcellulose and polyvinylpyrrolidone mixed in ratios, HPMCconc/PVPconc = 0.1 to 2.0 with a total polymer concentration of ~ 1.0–4.0% providing a viscosity of ~ 30–65 cps. A formulation of timolol maleate was prepared by combining buffers (borate) and preservative cations (Zn+2) with GFR to form an anionic polymer interpenetrating network (IPN) which obstructs the diffusion of the cationic timolol maleate, thus providing sustained action.
Results
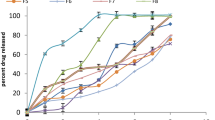
The GFR composition was optimized to impart physicochemical properties that were similar to artificial tear fluid, including clarity, with optical transmission of > 95%, adjusted osmolality of ~ 300 mOsmol, pH range of 6.5–7.5, surface tension of 35–42 mN/s, and 40–45 mPas viscosity for ocular retention and retarding lacrimal drainage. The mucoadhesive index of ~ 9.0 which was more than conventional timolol maleate eye drops further helped in eye drop retention. The solution was weakly pseudoplastic having a calculated blink viscosity of < 33 mPas for ensuring maximum lubrication and precluding the feeling of drag to the eyelid blink or eyeball movement. The formulation tested on rabbit eyes showed low timolol plasma levels and comparable ocular tissue levels and intraocular pressure reduction compared to the different marketed OD ophthalmic formulations.
Conclusion
The results indicated the GFR composition imparted acceptable physicochemical properties similar to artificial tear fluid. In vivo study on rabbit eyes showed comparable aqueous humor levels compared to the commercial timolol OD formulations.





Similar content being viewed by others
Availability of Data and Material
All data generated or analyzed during this study are included in this published article.
Code Availability
Not applicable.
References
Negri L, Ferreras A, Iester M. Timolol 0.1% in glaucomatous patients: efficacy, tolerance, and quality of life. J Ophthalmol. 2019. https://doi.org/10.1155/2019/4146124.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311(18):1901–11.
Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging. 2005;22(1):1–21.
Hoyng PF, Beek LMV. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59(3):411–34.
Pack insert timoptic, NDA no. 018086. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/018086s076lbl.pdf. Accessed 17 February 2021.
Davies NM, Farr FJ, Hadgraft J, Kellaway IW. Evaluation of mucoadhesive polymers in ocular drug delivery. I Viscous solutions Pharm Res. 1991;8:1039–43.
Kholdebarin R, Campbell RJ, ** YP, Buys YM. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008;43(4):454–6.
Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–40.
Tsai JC. A comprehensive perspective on patient adherence to topical glaucoma therapy. Ophthalmology. 2009;116(11):S30–6.
Shedden AH, Laurence J, Barrish A, Olah TV. Plasma timolol concentrations of timolol maleate: timolol gel-forming solution (timoptic XE) once daily versus timolol maleate ophthalmic solution twice daily. Doc Ophthalmol. 2001;103:73–9.
Schedden AH. Timolol maleate in gel-forming solution, a novel formulation of timolol maleate. Chibert Int J Ophthalmol. 1994;10:332–6.
Stewart WC, Sharpe ED, Stewart JA, Hott CE. The safety and efficacy of timolol 0.5% in xanthan gum versus timolol gel forming solution 0.5%. Curr Eye Res. 2002;24(5):389–91.
Yamamoto T, Kitazawa Y, Azuma I, Tsukahara S, Nakashima M. Clinical evaluation of a new formula of timolol maleate (WP-934 ophthalmic solution) W-934 Study group. Jpn J Ophthlamol. 1997;41:244–50.
Pack insert timolol maleate ophthalmic gel forming solution, NDA 20–330/S-027. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020330s027lbl.pdf. Accessed 18 February 2021.
Ohe NV, Stark M, Mayer H, Brewit H. How can the bioavailability of timolol be enhanced? A pharmacokinetic pilot study of novel hydrogels. Graefe’s Arch Clin Exp Ophthalmol. 1996;234:452–6.
Thermes F, Rozior A, Plazonnet B, Grove J. Bioadhesion: the effect of polyacrylic acid on the ocular bioavailability of Timolol. Int J Pharm. 1992;81:59–65.
Higashiyama M, Inada K, Ohtori A, Tojo K. Improvement of the ocular bioavailability of timolol by sorbic acid. Int J Pharm. 2004;272:91–8.
Rahi´c O, Tucak A, Omerovi´c N, Sirbubalo M, Hindija L, Hadžiabdi´c J, Vrani´c E. Novel drug delivery systems fighting glaucoma: formulation obstacles and solutions. Pharmaceutics. 2021;13:28.
Hassan EE, Gallo JM. Simple rheological method for the in-vitro assessment of mucin polymer bioadhesive bond strength. Pharm Res. 1990;79:491–5.
Karavas E, Georgarakis E, Bikiaris D. Application of PVP/HPMC miscible blends with enhanced mucoadhesive properties of adjusting drug release in predictable pulsatile choronotherapeutics. Eur J Pharm Biopharm. 2006;64:115–26.
Deslouis C, Tribollet B. Steady and modulated flow of an Ostwald fluid around a rotating disk. Rheol Acta. 1987;26(4):336–42.
Adewale FJ, Lucky AP, Oluwabunm AP, Boluwaji EF. Selecting the most appropriate model for rheological characterization of synthetic based drilling mud. Int J App Eng Res. 2017;12(18):7614–29.
Oechsner M, Keipert S. Polyacrylic acid/polyvinylpyrrolidone bipolymeric systems. I. Rheological and mucoadhesive properties of formulations potentially useful for the treatment of dry-eye-syndrome. Eur J Pharm Biopharm. 1999;47:113–8.
Peppas NA. Nanoscale technology of mucoadhesive interactions. Adv Drug Deliv Rev. 2004;56:1675–87.
Lu C, Konstanski L, Ketelson H, Meadows D, Pelton R. Hydroxy guar-borate interactions with tear film mucin and lysozyme. Langmuir. 2005;21:10032–7.
Eleftheriadis H, Liu C. Corneal endothelial cell destruction by intraocular use of benzalkonium chloride. J Cataract Refract Surg. 2002;28(9):1502–3.
Abelson MB, Udell IJ, Weston JH. Normal human tear pH by direct measurement. Arch Ophthalmol. 1981;99:301.
Tomilson A, Khanal S, Ramesh K, Diaper C. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invt Ophtalmol Vis Sci. 2006;47(10):4309–15.
Tiffany JM, Winter N, Bliss G. Tear film stability and tear surface tension. Curr Eye Res. 1989;8(5):507–15.
Chasonivkova LV, Formazyuk VE, Sergienko VI, Maichuk YF. Surface activity and wetting effect of artificial tear preparations. Bull Exper Biol Med. 1993;115(4):387–9.
Tiffany JM. The viscosity of human tears. Int Ophthalmol. 1991;15(6):371–6.
Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol in rabbits. J Pharm Sci. 1975;64:1312–6.
Leier CV, Baker ND, Weber PA. Cardiovascular effects of ophthalmic timolol. Ann Int Medicine. 1986;104:197–9.
Acknowledgements
The authors would like to thank Mr. Murlidhar Zope, Sun Pharma Advanced Research Company Limited, Tandalja, Vadodara, Gujarat, India, for the analytical support during the study. The authors thank Dr. Renuka Mishra of Scissential for the editorial assistance with the manuscript, which was funded by Sun Pharma Advanced Research Company Ltd.
Funding
The study was funded in the form of research grants from Sun Pharma Advanced Research Company Limited, Tandalja, Vadodara, Gujarat, India.
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, participated in drafting or revising the article, have agreed on the journal to which the article will be submitted, and give final approval of the version to be published.
Corresponding author
Ethics declarations
Ethics Approval
The research protocol was approved in the 17th and 23rd Institutional Animal Ethics Committee (IAEC) of Sun Pharma Advanced Research Company Ltd, Vadodara (Project numbers IACE#169, IACE# 174, and IACE#214).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
Arindam Halder, Malay D Shah, Bharat Pateliya, Vinod Burade, Ajay J. Khopade are employees of Sun Pharma Advanced Research Company Ltd, which funded this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Halder, A., Shah, M.D., Pateliya, B. et al. A Gel-Free Reservoir System for Once-a-Day Ophthalmic Delivery of Timolol Maleate. J Pharm Innov 17, 1247–1258 (2022). https://doi.org/10.1007/s12247-021-09601-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12247-021-09601-1