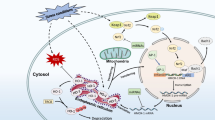
Abstract
Iron homeostasis and erythropoiesis are strongly interconnected. On one side iron is essential to terminal erythropoiesis for hemoglobin production, on the other erythropoiesis may increase iron absorption through the production of erythroferrone, the erythroid hormone that suppresses hepcidin expression Also erythropoietin production is modulated by iron through the iron regulatory proteins-iron responsive elements that control the hypoxia inducible factor 2-α. The second transferrin receptor, an iron sensor both in the liver and in erythroid cells modulates erythropoietin sensitivity and is a further link between hepcidin and erythropoiesis. When erythropoietin is decreased in iron deficiency the erythropoietin sensitivity is increased because the second transferrin receptor is removed from cell surface. A deranged balance between erythropoiesis and iron/hepcidin may lead to anemia, as in the case of iron deficiency, defective iron uptake and erythroid utilization or subnormal recycling. Defective control of hepcidin production may cause iron deficiency, as in the recessive disorder iron refractory iron deficiency anemia or in anemia of inflammation, or in iron loading anemias, which are characterized by excessive but ineffective erythropoiesis. The elucidation of the mechanisms that regulates iron homeostasis and erythropoiesis is leading to the development of drugs for the benefit of both iron and erythropoiesis disorders.

Similar content being viewed by others
References
Parrow NL, Li Y, Feola M, Guerra A, Casu C, Prasad P, et al. Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood. 2019;134(17):1373–84.
Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168(3):344–61.
Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3.
Aschemeyer S, Qiao B, Stefanova D, Valore EV, Sek AC, Ruwe TA, et al. Structure-function analysis of ferroportin defines the binding site and an alternative mechanism of action of hepcidin. Blood. 2018;131(8):899–910.
Billesbølle CB, Azumaya CM, Kretsch RC, Powers AS, Gonen S, Schneider S, et al. Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Nature. 2020;586(7831):807–11.
Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159–66.
Schwartz AJ, Das NK, Ramakrishnan SK, Jain C, Jurkovic MT, Wu J, et al. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J Clin Invest. 2019;129(1):336–48.
Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832–43.
Ganz T. Anemia of inflammation. N Engl J Med. 2019;381(12):1148–57.
Maio N, Rouault TA. Outlining the complex pathway of mammalian Fe-S cluster biogenesis. Trends Biochem Sci. 2020;45(5):411–26.
Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, et al. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood. 2007;110(4):1353–8.
Ducamp S, Fleming MD. The molecular genetics of sideroblastic anemia. Blood. 2019;133(1):59–69.
Miyajima H, Hosoi Y. Aceruloplasminemia. GeneReviews 2003. London: Intech; 2018.
Wang C-Y, Babitt JL. Liver iron sensing and body iron homeostasis. Blood. 2019;133(1):18–29.
Enns CA, Jue S, Zhang A-S. The ectodomain of matriptase-2 plays an important nonproteolytic role in suppressing hepcidin expression in mice. Blood. 2020;136(8):989–1001.
Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–11.
Colucci S, Pagani A, Pettinato M, Artuso I, Nai A, Camaschella C, et al. The immunophilin FKBP12 inhibits hepcidin expression by binding the BMP type I receptor ALK2 in hepatocytes. Blood. 2017;130(19):2111–20.
Du X, She E, Gelbart T, Truksa J, Lee P, **a Y, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–92.
Finberg KE, Heeney MM, Campagna DR, Aydinok Y, Pearson HA, Hartman KR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–71.
Camaschella C, Pagani A. Mendelian inheritance of anemia due to disturbed iron homeostasis. Semin Hematol. 2021;58(3):175–81.
De Falco L, Sanchez M, Silvestri L, Kannengiesser C, Muckenthaler MU, Iolascon A, et al. Iron refractory iron deficiency anemia. Haematologica. 2013;98(6):845–53.
Heeney MM, Finberg KE. Iron-refractory iron deficiency anemia (IRIDA). Hematol Oncol Clin N Am. 2014;28(4):637–52.
Bell S, Rigas AS, Magnusson MK, Ferkingstad E, Allara E, Bjornsdottir G, et al. A genome-wide meta-analysis yields 46 new loci associating with biomarkers of iron homeostasis. Commun Biol. 2021;4(1):156.
Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50.
Camaschella C, Girelli D. The changing landscape of iron deficiency. Mol Aspects Med. 2020;75: 100861.
Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46(7):678–84.
Arezes J, Foy N, McHugh K, Sawant A, Quinkert D, Terraube V, et al. Erythroferrone inhibits the induction of hepcidin by BMP6. Blood. 2018;132(14):1473–7.
Ganz T, Jung G, Naeim A, Ginzburg Y, Pakbaz Z, Walter PB, et al. Immunoassay for human serum erythroferrone. Blood. 2017;130(10):1243–6.
Camaschella C, Nai A. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. Br J Haematol. 2016;172(4):512–23.
Cazzola M. Ineffective erythropoiesis and its treatment. Blood. 2022;139(16):2460–70.
Kautz L, Jung G, Du X, Gabayan V, Chapman J, Nasoff M, et al. Erythroferrone contributes to hepcidin suppression and iron overload in a mouse model of β-thalassemia. Blood. 2015;126(17):2031–7.
Coffey R, Jung G, Olivera JD, Karin G, Pereira RC, Nemeth E, et al. Erythroid overproduction of erythroferrone causes iron overload and developmental abnormalities in mice. Blood. 2022;139(3):439–51.
Babitt JL. Erythroferrone in iron regulation and beyond. Blood. 2022;139(3):319–21.
Castro-Mollo M, Gera S, Ruiz-Martinez M, Feola M, Gumerova A, Planoutene M, et al. The hepcidin regulator erythroferrone is a new member of the erythropoiesis-iron-bone circuitry. Elife. 2021;10: e68217.
Nai A, Lidonnici MR, Rausa M, Mandelli G, Pagani A, Silvestri L, et al. The second transferrin receptor regulates red blood cell production in mice. Blood. 2015;125(7):1170–9.
Trombini P, Coliva T, Nemeth E, Mariani R, Ganz T, Biondi A, et al. Effects of plasma transfusion on hepcidin production in human congenital hypotransferrinemia. Haematologica. 2007;92(10):1407–10.
Bartnikas TB, Andrews NC, Fleming MD. Transferrin is a major determinant of hepcidin expression in hypotransferrinemic mice. Blood. 2011;117(2):630–7.
Kawabata H, Yang R, Hirama T, Vuong PT, Kawano S, Gombart AF, et al. Molecular cloning of transferrin receptor 2: a new member of the transferrin receptor-like family. J Biol Chem. 1999;274(30):20826–32.
Camaschella C, Roetto A, Calì A, De Gobbi M, Garozzo G, Carella M, et al. The gene TFR2 is mutated in a new type of haemochromatosis map** to 7q22. Nat Genet. 2000;25(1):14–5.
Nemeth E, Roetto A, Garozzo G, Ganz T, Camaschella C. Hepcidin is decreased in TFR2 hemochromatosis. Blood. 2005;105(4):1803–6.
Girelli D, Trombini P, Busti F, Campostrini N, Sandri M, Pelucchi S, et al. A time course of hepcidin response to iron challenge in patients with HFE and TFR2 hemochromatosis. Haematologica. 2011;96(4):500–6.
Forejtnikovà H, Vieillevoye M, Zermati Y, Lambert M, Pellegrino RM, Guihard S, et al. Transferrin receptor 2 is a component of the erythropoietin receptor complex and is required for efficient erythropoiesis. Blood. 2010;116(24):5357–67.
Rauner M, Baschant U, Roetto A, Pellegrino RM, Rother S, Salbach-Hirsch J, et al. Transferrin receptor 2 controls bone mass and pathological bone formation via BMP and Wnt signaling. Nat Metab. 2019;1(1):111–24.
Beguin Y. The soluble transferrin receptor: biological aspects and clinical usefulness as quantitative measure of erythropoiesis. Haematologica. 1992;77(1):1–10.
Pagani A, Vieillevoye M, Nai A, Rausa M, Ladli M, Lacombe C, et al. Regulation of cell surface transferrin receptor-2 by iron-dependent cleavage and release of a soluble form. Haematologica. 2015;100(4):458–65.
West AP Jr, Bennett MJ, Sellers VM, Andrews NC, Enns CA, Bjorkman PJ. Comparison of the interactions of transferrin receptor and transferrin receptor 2 with transferrin and the hereditary hemochromatosis protein HFE. J Biol Chem. 2000;275(49):38135–8.
Gruszczyk J, Huang RK, Chan L-J, Menant S, Hong C, Murphy JM, et al. Cryo-EM structure of an essential Plasmodium vivax invasion complex. Nature. 2018;559(7712):135–9.
Montemiglio LC, Testi C, Ceci P, Falvo E, Pitea M, Savino C, et al. Cryo-EM structure of the human ferritin-transferrin receptor 1 complex. Nat Commun. 2019;10(1):1121.
Jabara HH, Boyden SE, Chou J, Ramesh N, Massaad MJ, Benson H, et al. A missense mutation in TFRC, encoding transferrin receptor 1, causes combined immunodeficiency. Nat Genet. 2016;48(1):74–8.
Levy JE, ** O, Fujiwara Y, Kuo F, Andrews N. Transferrin receptor is necessary for development of erythrocytes and the nervous system. Nat Genet. 1999;21(4):396–9.
Wallace DF, Summerville L, Lusby PE, Subramaniam VN. First phenotypic description of transferrin receptor 2 knockout mouse, and the role of hepcidin. Gut. 2005;54(7):980–6.
Roetto A, Di Cunto F, Pellegrino RM, Hirsch E, Azzolino O, Bondi A, et al. Comparison of 3 Tfr2-deficient murine models suggests distinct functions for Tfr2-α and Tfr2-β isoforms in different tissues. Blood. 2010;115(16):3382–9.
Fillebeen C, Charlebois E, Wagner J, Katsarou A, Mui J, Vali H, et al. Transferrin receptor 1 controls systemic iron homeostasis by fine-tuning hepcidin expression to hepatocellular iron load. Blood. 2019;133(4):344–55.
Artuso I, Lidonnici MR, Altamura S, Mandelli G, Pettinato M, Muckenthaler MU, et al. Transferrin receptor 2 is a potential novel therapeutic target for β-thalassemia: evidence from a murine model. Blood. 2018;132(21):2286–97.
Casu C, Pettinato M, Liu A, Aghajan M, Lo Presti V, Lidonnici MR, et al. Correcting β-thalassemia by combined therapies that restrict iron and modulate erythropoietin activity. Blood. 2020;136(17):1968–79.
Guerra A, Parrow N, Mc Veigh P, Fleming R, Ginzburg Y, Rivella S. Obligate N-terminal but not C-terminal monoferric transferrin ameliorates anemia in β-Thalassemic mice. Blood. 2021;138(suppl 1):937.
Mirciov CSG, Wilkins SJ, Hung GCC, Helman SL, Anderson GJ, Frazer DM. Circulating iron levels influence the regulation of hepcidin following stimulated erythropoiesis. Haematologica. 2018;103(10):1616–26.
Artuso I, Pettinato M, Nai A, Pagani A, Sardo U, Billoré B, et al. Transient decrease of serum iron after acute erythropoietin treatment contributes to hepcidin inhibition by ERFE in mice. Haematologica. 2014;104(3):e87-90.
Nai A, Rubio A, Campanella A, Gourbeyre O, Artuso I, Bordini J, et al. Limiting hepatic Bmp-Smad signaling by matriptase-2 is required for erythropoietin-mediated hepcidin suppression in mice. Blood. 2016;127(19):2327–36.
Li H, Rybicki AC, Suzuka SM, von Bonsdorff L, Breuer W, Hall CB, et al. Transferrin therapy ameliorates disease in β-thalassemic mice. Nat Med. 2010;16(2):177–82.
Casu C, Chessa R, Liu A, Gupta R, Drakesmith H, Fleming R, et al. Minihepcidins improve ineffective erythropoiesis and splenomegaly in a new mouse model of adult β-thalassemia major. Haematologica. 2020;105(7):1835–44.
Guo S, Casu C, Gardenghi S, Booten S, Aghajan M, Peralta R, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123(4):1531–41.
Arezes J, Foy N, McHugh K, Quinkert D, Benard S, Sawant A, et al. Antibodies against the erythroferrone N-terminal domain prevent hepcidin suppression and ameliorate murine thalassemia. Blood. 2020;135(8):547–57.
Manolova V, Nyffenegger N, Flace A, Altermatt P, Varol A, Doucerain C, et al. Oral ferroportin inhibitor ameliorates ineffective erythropoiesis in a model of β-thalassemia. J Clin Invest. 2019;130(1):491–506.
Cappellini MD, Viprakasit V, Taher AT, Georgiev P, Kuo KHM, Coates T, et al. A phase 3 trial of luspatercept in patients with transfusion-dependent β-Thalassemia. N Engl J Med. 2020;382(13):1219–31.
Eckardt K-U, Agarwal R, Aswad A, Awad A, Block GA, Bacci MR, et al. Safety and efficacy of vadadustat for anemia in patients undergoing dialysis. N Engl J Med. 2021;384(17):1601–12.
Chertow GM, Pergola PE, Farag YMK, Agarwal R, Arnold S, Bako G, et al. Vadadustat in patients with anemia and non–dialysis-dependent CKD. N Engl J Med. 2021;384(17):1589–600.
Ghosh MC, Zhang D-L, Jeong SY, Kovtunovych G, Ollivierre-Wilson H, Noguchi A, et al. Deletion of iron regulatory protein 1 causes polycythemia and pulmonary hypertension in mice through translational derepression of HIF2α. Cell Metab. 2013;17(2):271–81.
Anderson SA, Nizzi CP, Chang Y-I, Deck KM, Schmidt PJ, Galy B, et al. The IRP1-HIF-2α axis coordinates iron and oxygen sensing with erythropoiesis and iron absorption. Cell Metab. 2013;17(2):282–90.
Ghosh MC, Zhang D-L, Ollivierre WH, Noguchi A, Springer DA, Linehan WM, et al. Therapeutic inhibition of HIF-2α reverses polycythemia and pulmonary hypertension in murine models of human diseases. Blood. 2021;137(18):2509–19.
Zhang D-L, Wu J, Shah BN, Greutélaers KC, Ghosh MC, Ollivierre H, et al. Erythrocytic ferroportin reduces intracellular iron accumulation, hemolysis, and malaria risk. Science. 2018;359(6383):1520–3.
Zhang D-L, Ghosh MC, Ollivierre H, Li Y, Rouault TA. Ferroportin deficiency in erythroid cells causes serum iron deficiency and promotes hemolysis due to oxidative stress. Blood. 2018;132(19):2078–87.
Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–9.
Nai A, Lidonnici MR, Federico G, Pettinato M, Olivari V, Carrillo F, et al. NCOA4-mediated ferritinophagy in macrophages is crucial to sustain erythropoiesis in mice. Haematologica. 2021;106(3):795–805.
Ryu M-S, Zhang D, Protchenko O, Shakoury-Elizeh M, Philpott CC. PCBP1 and NCOA4 regulate erythroid iron storage and heme biosynthesis. J Clin Invest. 2017;127(5):1786–97.
Mancias JD, PontanoVaites L, Nissim S, Biancur DE, Kim AJ, Wang X, et al. Ferritinophagy via NCOA4 is required for erythropoiesis and is regulated by iron dependent HERC2-mediated proteolysis. Elife. 2015;4: e10308.
Santana-Codina N, Gableske S, Quiles del Rey M, Małachowska B, Jedrychowski MP, Biancur DE, et al. NCOA4 maintains murine erythropoiesis via cell autonomous and non-autonomous mechanisms. Haematologica. 2019;104(7):1342–54.
Das NK, Jain C, Sankar A, Schwartz AJ, Santana-Codina N, Solanki S, et al. Modulation of the HIF2α-NCOA4 axis in enterocytes attenuates iron loading in a mouse model of hemochromatosis. Blood. 2022;139(16):2547–52.
Xavier-Ferrucio J, Scanlon V, Li X, Zhang P-X, Lozovatsky L, Ayala-Lopez N, et al. Low iron promotes megakaryocytic commitment of megakaryocytic-erythroid progenitors in humans and mice. Blood. 2019;134(18):1547–57.
Di Buduo CA, Currao M, Pecci A, Kaplan DL, Balduini CL, Balduini A. Revealing eltrombopag’s promotion of human megakaryopoiesis through AKT/ERK-dependent pathway activation. Haematologica. 2016;101(12):1479–88.
Liu Z-J, Deschmann E, Ramsey HE, Feldman HA, Psaila B, Cooper N, et al. Iron status influences the response of cord blood megakaryocyte progenitors to eltrombopag in vitro. Blood Adv. 2022;6(1):13–27.
Funding
This work was supported by European Hematology Association (Advanced Research Grant 2020) and Italian Ministry of Health (GR-2019-12369583) to A.N
Author information
Authors and Affiliations
Contributions
CC conceived the structure of the paper. AP, LS and AN contributed to writing. LS contributed the figure. All authors revised and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
A.N. received research funding from Celgene (BMS group). The other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Camaschella, C., Pagani, A., Silvestri, L. et al. The mutual crosstalk between iron and erythropoiesis. Int J Hematol 116, 182–191 (2022). https://doi.org/10.1007/s12185-022-03384-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12185-022-03384-y