Abstract
Background
Sorafenib is a standard treatment for patients (pts) with advanced hepatocellular carcinoma (aHCC), although the clinical benefit is heterogeneous between different pts groups. Among novel prognostic factors, a low baseline neutrophil-to-lymphocyte ratio (bNLR) and early-onset diarrhoea have been linked with a better prognosis.
Purpose
To identify prognostic factors in pts with aHCC treated with 1st-line sorafenib and to develop a new prognostic score to guide management.
Materials and methods
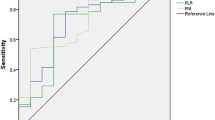
Retrospective review of 145 pts bNLR, overall toxicity, early toxicity rates and overall survival (OS) were assessed. Univariate and multivariate analysis of prognostic factors for OS was performed. The prognostic score was calculated from the coefficients found in the Cox analysis. ROC curves and pseudoR2 index were used for internal validation. Discrimination ability and calibration were tested by Harrel’s c-index (HCI) and Akaike criteria (AIC).
Results
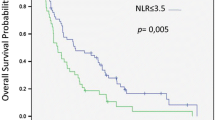
The optimal bNLR cut-off for the prediction of OS was 4 (AUC 0.62). Independent prognostic factors in multivariate analysis for OS were performance status (PS) (p < .0001), Child–Pugh (C–P) score (p = 0.005), early-onset diarrhoea (p = 0.006) and BNLR (0.011). The prognostic score based on these four variables was found efficient (HCI = 0.659; AIC = 1.180). Four risk groups for OS could be identified: a very low-risk (median OS = 48.6 months), a low-risk (median OS = 11.6 months), an intermediate-risk (median OS = 8.3 months) and a high-risk group (median OS = 4.4 months).
Conclusions
PS and C–P score were the main prognostic factors for OS, followed by early-onset diarrhoea and bNLR. We identified four risk groups for OS depending on these parameters. This prognostic model could be useful for patient stratification, but an external validation is needed.

Similar content being viewed by others
References
Globocan. Estimated cancer incidence mapwiLc. 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. 24th Jan 2014.
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34.
Lencioni R, Marrero J, Venook A, Ye SL, Kudo M. Design and rationale for the non-interventional global investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034–41.
Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–63.
Imedio ER, Beveridge RD, Urtasun JA, Campos GB, Estellés DL, Esparcia MF, et al. Safety and efficacy of sorafenib in the treatment of advanced hepatocellular carcinoma: a single center experience. Med Oncol. 2014;31:948–54.
Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66.
Okuda K, Ohtsuki T, Obata H. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Cancer. 1985;56:918–28.
Llovet JM, Bruix J. Prospective validation of the Cancer of the Liver Italian Program (CLIP) score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;32:679–80.
Chan SL, Mo FK, Johnson PJ. Prospective validation of the Chinese University Prognostic Index and comparison with other staging systems for hepatocellular carcinoma in an Asian population. J Gastroenterol Hepatol. 2011;26:340–7.
Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patient with hepatocellular carcinoma. Groupe d’Étude et de Traitement du Carcinome Hepatocellulaire. J Hepatol. 1999;31:133–41.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38.
Chan AW, Chan RC, Wong GL, Wong VW, Choi PC, Chan HL, et al. New simple prognostic score for primary biliary cirrhosis: albumin-bilirubin score. J Gastroenterol Hepatol. 2015;30:1391–6.
Chung H, Kudo M, Takahashi S, Hagiwara S, Sakaguchi Y, Inoue T, et al. Comparison of three current staging systems for hepatocellular carcinoma: Japan integrated staging score, new Barcelona Clinic Liver Cancer staging classification, and Tokyo score. J Gastroenterol Hepatol. 2008;23:445–52.
Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385–92.
Shomura M, Kagawa T, Shiraishi K, Hirose S, Arase Y, Koizumi J, et al. Skin toxicity predicts efficacy to sorafenib in patients with advanced hepatocellular carcinoma. World J Hepatol. 2014;6:670–6.
Yada M, Masumoto A, Motomura K, Tajiri H, Morita Y, Suzuki H, et al. Indicators of sorafenib efficacy in patients with advanced hepatocellular carcinoma. World J Gastroenterol. 2014;20:12581–7.
Reig M, Torres F, Rodriguez-Lope C, Forner A, Llarch N, Rimola J, et al. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318–24.
da Fonseca LG, Barroso-Sousa R, Bento Ada S, Blanco BP, Valente GL, Pfiffer TE, et al. Pre-treatment neutrophil-to-lymphocyte ratio affects survival in patients with advanced hepatocellular carcinoma treated with sorafenib. Med Oncol. 2014;31:264–9.
Li X, Chen ZH, Ma XK, Chen J, Wu DH, Lin Q, et al. Neutrophil-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2014;35:11057–63.
Wei K, Wang M, Zhang W, Mu H, Song T-Q, Song TQ. Neutrophil-lymphocyte ratio as a predictor of outcomes for patients with hepatocellular carcinoma undergoing TAE combined with sorafenib. Med Oncol. 2014;31:969–74.
Sukato DC, Tohme S, Chalhoub D, Han K, Zajko A, Amesur N, et al. The prognostic role of neutrophil-to-lymphocyte ratio in patients with unresectable hepatocellular carcinoma treated with radioembolization. J Vasc Interv Radiol. 2015;26:816–24.
Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;15:2290–300.
Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, et al. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013;14:5527–31.
Ji F, Lian Y, Fu SH, Guo ZY, Shu M, Shen SL, et al. A novel and accurate predictor of survival for patients with hepatocellular carcinoma after surgical resection: the neutrophil to lymphocyte ratio (NLR) combined with the aspartate aminotransferase/platelet count ratio index (APRI). BMC Cancer. 2016;16:137–48.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Cancer Rev. 2016;16:431–46.
Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: primed to make a difference? Cancer. 2016;122:367–77.
Tsuchiya N, Sawada Y, Endo I, Uemura Y, Nakatsura T. Potentiality of immunotherapy against hepatocellular carcinoma. World J Gastroenterol. 2015;21:10314–26.
Lamarca A, Abdel-Rahman O, Salu I, McNamara MG, Valle JW, Hubner RA. Identification of clinical biomarkers for patients with advanced hepatocellular carcinoma receiving sorafenib. Clin Transl Oncol. 2017;9:364–72.
Zugazagoitia J, Manzano A, Sastre J, Ladero JM, Puente J, Díaz-Rubio E. Sorafenib for non-selected patient population with advanced hepatocellular carcinoma: efficacy and safety data according to liver function. Clin Transl Oncol. 2013;15:146–53.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent is not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Diaz-Beveridge, R., Bruixola, G., Lorente, D. et al. An internally validated new clinical and inflammation-based prognostic score for patients with advanced hepatocellular carcinoma treated with sorafenib. Clin Transl Oncol 20, 322–329 (2018). https://doi.org/10.1007/s12094-017-1720-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-017-1720-4