Abstract
To evaluate the efficacy and safety of early steroid withdrawal or steroid avoidance in the tacrolimus (Tac)-based immunosuppressive regimen for liver transplant recipients. According to the requirements of the Cochrane systematic review, a thorough literature search was performed in the PubMed/MEDLINE and Cochrane electronic databases between 1995 and 2011 using the key words “liver transplantation,” “Tac,” and “steroid free” or “steroid withdrawal,” restricting articles to the English language. Data were processed for a meta-analysis by Stata 12 software. Altogether 17 prospective randomized controlled trials containing 1,980 transplanted patients were included in this study. The overall pooled RR estimates of 1-, 2-, 3-, and 5-year patient and graft survival rates were 0.985, 0.998, 0.995, and 1.100 (95 % CI 0.925–1.048, 0.934–1.067, 0.894–1.107, and 0.968–1.250, respectively), as well as 0.998, 0.993, 0.945, and 1.053, respectively (95 % CI 0.928–1.072, 0.902–1.092, 0.833–1.072, and 0.849–1.307, respectively). The other pooled RR estimates of acute rejection and chronic rejection rates for all enrolled studies were 1.077 and 0.311 (95 % CI 0.864–1.343 and 0.003–37.207). As for secondary predictors, the pooled RR estimates such as HCV recurrence, HCC recurrence, diabetes, hypertension, kidney dysfunction, bacterial infection, and CMV were 1.101, 1.403, 1.836, 1.607, 0.842, 1.096, and 2.280, respectively (95 % CI 0.964–1.257, 0.422–4.688, 1.294–2.606, 0.926–1.228, 0.693–1.022, 0.783–1.533, and 1.500–3.465, respectively). There were no differences between the steroid group and steroid-free group for all clinical observational indices except for the incidence of diabetes (p = 0.001) and CMV infection (p < 0.001). In summary, our study indicate that rapid discontinuation of steroid in the Tac-based immunosuppressive regimen may not lead to an increased risk of morbidity and rejection rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Orthotopic liver transplantation (OLT) has been recognized as a well-established therapeutic option for a subset of patients with benign end-stage liver diseases as well as early stage hepatocellular carcinoma (HCC), achieving a favorable long-term survival rate in many liver transplant centers in recent years [1]. It must be admitted that the success of OLT is owed to the pioneers develo** the surgical procedures and to the researchers discovering the available medications related to allograft rejection prevention [2]. Although liver allograft is generally considered immunologically privileged, and hyperacute rejection is rarely observed, the substantial short- and long-term morbidity associated with acute and chronic rejection have still set off a wave of investigators seeking a safe and effective immunosuppressive regimen for liver transplant recipients [3, 4].
Steroids have long been recognized as part of the immunosuppressive regimen for induction and maintenance since the advent of clinical OLT [5]. Boluses of high-dose steroids are routinely administered during and after the operation for the control of acute cellular rejection in many liver transplant centers. However, prolonged use of steroids is associated with multiple severe side effects including hypertension, hyperlipidemia, obesity, diabetes mellitus, osteoporosis, infectious complications, and particularly growth retardation in children [6]. In addition, hepatitis C virus (HCV) and tumor recurrence upon OLT should also be taken into consideration when patients are exposed to high-dose long-term steroids [7–10]. In such circumstances, Pirenne et al. reported the long-term (median = 40 months) follow-up data of a prospective study designed to determine whether OLT could be performed with no steroids at all. This prospective single-center pilot study showed that OLT without steroids is feasible and yields no penalty in terms of acute and chronic rejection, immune graft loss, graft function, patient and graft survival [11]. An experience from Germany with about 30 adult liver graft recipients subjected to dual maintenance immunosuppression consisting of tacrolimus (Tac) and mycophenolate mofetil (MMF) without prophylactic steroids revealed that patient and graft survival at 2 years was 86.7 and 83.9 %, respectively [12]. All rejections were completely reversible by temporary addition of steroids. Therefore, the authors speculated that double drug immunosuppression with Tac and MMF is effective and safe in terms of patient and graft survival as well as incidence and severity of rejection [12]. In addition, close drug monitoring is advised after OLT in order to avoid under- or over-immunosuppression, which may be caused by impaired absorption or metabolism [12]. Thus, minimization of steroid usage including steroid-sparing or steroid-free immunosuppressive regimens seems to be the pursued goal for all liver transplant experts to achieve better outcomes [13–16].
However, several pilot studies and a few randomized trials have explored this possibility with mixed results. Reggiani et al. [17] performed a single-center, randomized, 1:1, open-label, controlled study and speculated that a primary immunosuppressive regimen based on Tac and low-dose MMF without steroids is safe but unable to prevent acute rejection at 1 week after transplantation even if early acute rejection does not affect the outcome in terms of morbidity and graft or patient survival. Foroncewicz et al. [18] in Poland conducted a 6-year, single-center, retrospective study including 25 liver transplant recipients. Though results indicated that a steroid-free regimen of Tac is as effective as Tac/steroid in achieving good patient and graft survival, no substantial benefits concerning the safety of Tac therapy were evident during long-term follow-up [18].
More recently, considering the potential detrimental effect on renal functions resulting from the usage of high-dose Tac instead of steroid, some induction agents for specific immunological tolerance including polyclonal rabbit antithymocyte globulin (RATG) and IL-2 receptor monoclonal antibody (basiliximab or daclizumab) have been suggested in triple or quadruple immunosuppressive protocols during OLT, which could minimize the use of Tac and limit renal toxicity [19–21]. To date, there is no consensus about the role of steroid minimization in the Tac-based immunosuppressive regimen for liver transplant recipients. The purpose of our study was to conduct a systematic review and meta-analysis of the published prospective randomized controlled trials since 1995 concerning the efficacy and safety of steroid elimination in a Tac-based immunosuppressive regimen for OLT patients.
Methods
This is a systematic review including a meta-analysis, which was performed according to the preferred reporting items for the systematic reviews and meta-analyses (PRISMA) statement [22] and the Cochrane Handbook for Systematic Reviews of Interventions [23].
Search strategy and selection criteria
Two authors (**yang Gu and Jun Li) independently searched the databases PubMed/MEDLINE and Cochrane Central Register for all levels of evidence from medical research articles published in print or electronically in English from 1995 to 2011. A global literature search was undertaken by combinations of the following search terms: “liver transplantation,” “Tac,” and “steroid free” or “steroid withdrawal” for the purpose of the role of steroid minimization in the Tac-based immunosuppressive regimen for liver transplant recipients.
The detailed inclusion criteria of trials were as follows: (1) to assure the quality of analysis, only randomized controlled trials were included in the study; (2) comparisons of outcomes were made between a Tac-based immunosuppressive regimen with (lasting time more than 3 months) or without steroid (lasting time within 3 months) for OLT; (3) if multiple publications reported estimates based on the same study population, the largest or most recent sample was used; (4) studies must have reported patient or graft survival rates, acute or chronic rejection prevalence, as well as complication incidence in relation to steroid usage; and (5) our search included only those original articles published in English.
Data extraction and outcome measures
Two investigators (**yang Gu and Jun Li) independently determined the eligibility of each publication for the systematic review and meta-analysis by filling in a Microsoft Excel spreadsheet and evaluating study quality, with disagreements resolved by a third reviewer (Jun Wang). Extracted data included general information (first author, year of publication, study center, and sample size), demographics of participants (gender ratio, mean age, concomitant disease, and MELD score), characteristics of clinical interventions (etiology distribution and immunosuppressive regimen), primary endpoints (survival rates and rejection rates), and secondary endpoints (complication incidences related to steroid usage) from the texts, tables, and graphs of published eligible trial reports. Pooled outcome measures for OLT in a Tac-based immunosuppressive regimen with or without steroid involved patient and graft survival rates at 1, 3, and 5 years, incidence of acute and chronic rejection, incidence of recurrent hepatitis C or HCC, rates of infectious complications, post-OLT metabolic disease occurrence, as well as kidney dysfunction.
Quality assessment
Two authors (**yang Gu and Jun Wang) independently assessed the methodological quality of the included trials using the quality checklist recommended by the Cochrane Handbook [24]. The following domains on the risk of bias were assessed: randomization, patients blinded, concealment of treatment allocation, intention-to-treat analysis, and incomplete outcome. We resolved all disagreements by discussion and referral to a third author (Jun Li) for adjudication.
Data synthesis and analysis
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [23]. Funnel plots and Egger’s tests were created using standard techniques for detecting publication bias. For randomized controlled trials, outcome data were pooled using a random effect model weighted by the inverse variance. The meta-analyses results of continuous variables were expressed as mean differences and as risk ratios (RR) for binary outcomes with 95 % CI. Meta-analyses of the binary variables were conducted on the log-odds ratios to satisfy the assumption of normality of effect sizes. Statistical analyses were performed using STATA 12. Instead, we undertook specific stratified meta-analyses to examine the sensitivity of the findings of the review to key potential causes of heterogeneity.
Publication bias
We assessed the potential for publication bias through visual inspection of funnel plot asymmetry and evaluated the statistical significance of differences among the including trials with Begg’s test.
Results
Literature search results
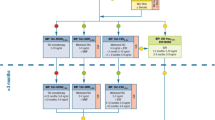
The electronic database searches yielded a total of 252 citations comprising 151 publications in PubMed and 101 in the Cochrane Central Register between 1995 and 2011. We identified 55 potentially relevant studies that were retrieved and reviewed by titles and abstracts, 25 of which were further excluded because of the absence of a control group or lack of a detailed outcome index. Of the 30 possible studies meeting our inclusion criteria, 13 duplicate papers derived from the same clinical centers were excluded from our present study, and we finally included 17 eligible full-text articles with the largest population and distinct observational index in this meta-analysis, which were further divided into two sections consisting of studies with comparison of Tac-based immunosuppressive regimens with steroids or not, as well as with induction agents or not. The flow chart of the search and selection is illustrated in Fig. 1.
Diagram of the literature search and selection process. A total of 252 citations comprising 151 publications in PubMed and 101 in the Cochrane Central Register were yielded between 1995 and 2011. We identified 55 potentially relevant studies that were retrieved and reviewed by titles and abstracts, 25 of which were further excluded because of the absence of a control group or lack of a detailed outcome index. Of the 30 possible studies meeting our inclusion criteria, 13 duplicate papers deriving from the same clinical centers were excluded, and finally 17 eligible full-text articles were included with the largest population and distinct observational index in this meta-analysis, which were further divided into two sections consisting of studies with comparison of Tac-based immunosuppressive regimens with steroids or not, as well as with induction agents or not
Characteristics of included studies
Study and patient characteristics are summarized in Tables 1 and 2. Overall, the 17 randomized, controlled trials enrolled a total of 1,980 participants with a mean age of 44.4 years, of which approximately 65 % were male. Five studies were based in the USA [26, 32, 35, 36, 38], three in Germany [25, 28, 31], three in Italy [17, 29, 34], and one each in Spain [27], China [9], UK [30], France [33], Belgium [37], and Poland [18]. The 17 prospective randomized controlled studies enrolled patients with distinct primary diseases eligible for OLT such as hepatitis B virus (HBV) [18, 27, 31–33], HCV infection [18, 25–27, 30–33, 35, 36, 38], HCC [9, 17, 26, 27, 30, 31, 33, 37], primary sclerosing cholangitis (PSC) or primary biliary cirrhosis (PBC) [17, 18, 31, 32], alcoholic cirrhosis [17, 18, 26, 27, 31], and autoimmune hepatitis (AIH) [28, 32]. A proportion of selected studies (6/17) described concomitant diseases such as diabetes [27, 29–31, 33], hypertension [27, 31], cytomegalovirus (CMV) infection [33, 34], Epstein-Barr virus (EBV) infection [33, 34], and metabolic disease [31, 33, 34, 37]. Of these 17 studies, all but 2 publications [28, 30] reported intraoperative steroid usage to avoid hyperacute rejection. As far as postoperative steroid duration was concerned, it was totally different among liver transplantation centers ranging from 3 to 72 months for the control group (steroid group) and <3 months for the experimental group (steroid-free group). The remnant observational index including time of Tac and MMF duration, and Tac blood level is displayed in detail in Table 2. The overall 17 prospective randomized controlled trials were then divided into two parts in terms of whether steroid was employed upon OLT or not (Sect. I), as well as whether induction agents were employed during OLT (Sect. II), which was further analyzed for all 17 trials and for each section, respectively. Tables 3 and 4 display a summary of outcomes including survival rates and complication incidence.
Quality assessment
We evaluated the quality of each trial according to the Jadad scale. Five domains were assessed: randomization, patient blinding, concealment of treatment allocation, intention-to-treat analysis, and incomplete outcome (Supplementary Table 1). All included articles described their study design as prospective randomized controlled trials. Only 11.8 % (2/17) reported patient blinding and concealed allocation [30, 31], and 23.5 % (4/17) used intention-to-treat analysis [26, 30, 33, 38]. In addition, the overwhelming majority of publications lacked complete outcome data except for two [27, 31].
Primary predictors
Supplementary Table 2 summarizes the meta-analysis results of pooled primary outcomes for all 17 enrolled RCTs in this study. The overall 1-, 2-, 3-, and 5-year pooled RR estimates of patient survival rates and graft survival rates were 0.985 (95 % CI 0.925–1.048), 0.998 (95 % CI 0.934–1.067), 0.995 (95 % CI 0.894–1.107), 1.100 (95 % CI 0.968–1.250), as well as 0.998 (95 % CI 0.928–1.072), 0.993 (95 % CI 0.902–1.092), 0.945 (95 % CI 0.833–1.072), and 1.053 (95 % CI 0.849–1.307), respectively (Fig. 2a, b). The other pooled RR estimates of acute rejection and chronic rejection rates for all enrolled studies were 1.077 (95 % CI 0.864–1.343) and 0.311 (95 % CI 0.003–37.207) (Fig. 2c). There were no differences between the steroid group and steroid-free group for the primary endpoints.
Forest plot of RR and 95 % CI for patient survival rates (a), graft survival rates (b), and rejection rates (c) and incidence of complications (d) for all 17 enrolled RCTs in this study. The horizontal lines represent the 95 % CI of the RR for the steroid group compared to steroid-free group in each study. The black box in the middle of the CI represents the single best estimate of RR in that study. The width of the CI is related to the power of the study and inversely associated with sample size. In addition, the pooled or combined RR results of the meta-analysis are represented by a diamond, the width of which is the CI for the pooled data. The vertical line is typically displayed to indicate no effect when RR = 1. When the CI crosses the vertical line of no effect, we must accept the null hypothesis of no difference between two groups. Only if the CI remains clear of the vertical line of no effect can we reject the null hypothesis and conclude that steroid minimization likely caused the outcome. We used a fixed effect model for meta-analysis, except that heterogeneity between studies was considered present if the p value was <0.1 or I 2 was more than 50 %, where we used a random effect model instead
The detailed pooled RR estimates of survival rates and rejection rates for Sects. I and II are listed in Supplementary Tables 3 and 4, respectively. In Sect. I, the overall 1-, 2-, 3-, and 5-year pooled RR estimates of patient survivals and graft survival rates were 0.988 (95 % CI 0.896–1.090), 1.032 (95 % CI 0.931–1.145), 1.021 (95 % CI 0.876–1.189), and 1.100 (95 % CI 0.968–1.250), as well as 0.991 (95 % CI 0.879–1.118), 1.013 (95 % CI 0.847–1.212), 0.905 (95 % CI 0.606–1.352), and 1.061 (95 % CI 0.855–1.316), respectively (Fig. 3a, b). The other pooled RR estimates of acute rejection and chronic rejection rates were 0.983 (95 % CI 0.774–1.247) and 0.126 (95 % CI 0.030–0.526) (Fig. 3c). In Sect. II, the overall 1- and 2-year pooled RR estimates of patient survival rates and graft survival rates were 0.982 (95 % CI 0.904–1.065) and 0.977 (95 % CI 0.895–1.067) as well as 1.005 (95 % CI 0.916–1.102) and 0.968 (95 % CI 0.863–1.085), respectively (Fig. 4a, b). The pooled RR estimate of acute rejection rates was 1.130 (95 % CI 0.927–1.377) (Fig. 4c). In general, steroid elimination and plus induction agent employment during OLT could achieve comparably favorable survival rates and rejection rates of no significance compared with traditional long-term steroid usage.
Forest plot of RR and 95 % CI for patient survival rates (a), graft survival rates (b), and rejection rates (c) and incidence of complications (d) for Sect. I in this study. The horizontal lines represent the 95 % CI of the RR for the steroid group compared to the steroid-free group in each study. The black box in the middle of the CI represents the single best estimate of RR in that study. The width of the CI is related to the power of the study and inversely associated with sample size. In addition, the pooled or combined RR results of the meta-analysis are represented by a diamond, the width of which is the CI for the pooled data. The vertical line is typically displayed to indicate no effect when RR = 1. When the CI crosses the vertical line of no effect, we must accept the null hypothesis of no difference between two groups. Only if the CI remains clear of the vertical line of no effect can we reject the null hypothesis and conclude that steroid minimization likely caused the outcome. We used a fixed effect model for meta-analysis, except that heterogeneity between studies was considered present if the p value was <0.1 or I 2 was more than 50 %, where we used a random effect model instead
Forest plot of RR and 95 % CI for patient survival rates (a), graft survival rates (b), and rejection rates (c) and incidence of complications (d) for Sect. II in this study. The horizontal lines represent the 95 % CI of the RR for the non-induction group compared to the induction group in each study. The black box in the middle of the CI represents the single best estimate of RR in that study. The width of the CI is related to the power of the study and inversely associated with sample size. In addition, the pooled or combined RR results of the meta-analysis are represented by a diamond, the width of which is the CI for the pooled data. The vertical line is typically displayed to indicate no effect when RR = 1. When the CI crosses the vertical line of no effect, we must accept the null hypothesis of no difference between two groups. Only if the CI remains clear of the vertical line of no effect can we reject the null hypothesis and conclude that the prescription of induction agents during steroid minimization likely caused the outcome. We used a fixed effect model for meta-analysis, except that heterogeneity between studies was considered present if the p value was <0.1 or I 2 was more than 50 %, where we used a random effect model instead
Secondary predictors
As shown in Supplementary Table 2 and Fig. 2d, the pooled RR estimates of secondary outcomes such as HCV recurrence (1.101; 95 % CI 0.964–1.257), HCC recurrence (1.403; 95 % CI 0.422–4.688), diabetes (1.836; 95 % CI 1.294–2.606), hypertension (1.607; 95 % CI 0.926–1.228), kidney dysfunction (0.842; 95 % CI 0.693–1.022), bacterial infection (1.096; 95 % CI 0.783–1.533), and CMV (2.280; 95 % CI 1.500–3.465) for all 17 RCTs were presented. Of note, the combined complication incidence estimates including diabetes (p = 0.001) and CMV (p < 0.001) were significantly reduced with early steroid withdrawal. In Sect. I, the pooled RR estimates of HCV recurrence (0.926; 95 % CI 0.586–1.463), HCC recurrence (2.437; 95 % CI 0.890–6.678), diabetes (1.223; 95 % CI 0.766–1.954), hypertension (0.975; 95 % CI 0.503–1.889), kidney dysfunction (0.807; 95 % CI 0.442–1.472), bacterial infection (0.529; 95 % CI 0.261–1.072), and CMV (2.137; 95 % CI 0.809–5.643) are displayed in Fig. 3d. No significance was observed between the steroid group and steroid-free group with respect to any complication incidence. In Sect. II, the pooled RR estimates of HCV recurrence (1.136; 95 % CI 0.993–1.300), diabetes (1.170; 95 % CI 1.093–1.252), hypertension (1.036; 95 % CI 0.980–1.095), kidney dysfunction (0.934; 95 % CI 0.869–1.004), bacterial infection (1.204; 95 % CI 0.941–1.541), and CMV (1.079; 95 % CI 0.968–1.203) are displayed in Fig. 4d. Compared with the non-induction group, the combined diabetes incidence estimates of the induction group were significantly decreased upon induction agent intervention (p < 0.001). The detailed pooled RR estimates of complication incidence for Sects. I and II are listed in Supplementary Tables 3 and 4, respectively.
Publication bias
The funnel plot did not show any asymmetrical pattern, and the Begg’s test did not reveal any significant publication bias (data not shown).
Discussion
To the best of our knowledge, our current study represents the first evidence-based medicine article concerning the efficacy and safety of steroid minimization in the Tac-based immunosuppressive regimen for liver transplant recipients. In this meta-analysis, altogether 17 clinical trials containing 1,980 transplanted patients published between 1995 and 2011 were finally enrolled in this study. To clarify whether induction agents could reduce potential adverse effects related to steroid avoidance by modulating the immunologic status, the enrolled studies were divided into two sections in terms of: (1) whether a steroid was employed upon OLT or not; (2) whether induction agents were employed during OLT or not. To our excitement, our results indicated that early steroid withdrawal or steroid avoidance in the Tac-based immunosuppressive regimen is safe and effective for the prevention of acute and chronic rejection after OLT with the benefit of a decrease in the incidence of diabetes and CMV infection. Although the underlying data remain to be systematically investigated, the present study revealed the introduction of some induction agents including RATG, basiliximab and daclizumab in triple and quadruple immunosuppressive protocols are likely more effective in lowering the high-dose usage of Tac in order to minimize the potential detrimental effect on renal functions. In the following paragraphs, some important issues pertaining to steroid elimination in the Tac-based immunosuppressive regimen for liver transplant recipients will be discussed.
Since the discovery of cortisol in 1937, steroids have paved the way for successful medical immunosuppression, especially for later organ transplantation, and proved the reversibility of rejection. However, in the modern era of an improved immunosuppressive protocol, it is necessary to critically assess the risk:benefit ratio of long-term steroid therapy during and after liver transplantation. To date, several studies have shown that weaning from steroids can be successfully carried out shortly after liver transplantation, thereby decreasing typical steroid-related side effects including new-onset diabetes mellitus, lipid metabolism abnormality, viral hepatitis recurrence and liver malignancy relapse [27, 29, 30, 35, 38]. In spite of these encouraging results, the theoretical advantages should be carefully balanced against the potential risks of increasing nonsteroidal immunosuppressive complications and a higher incidence of rejection. Jain et al. [39] reported 23.8 % of patients under the Tac-based immunosuppressive regimen required steroid reintroduction for late rejection, recurrence of the autoimmune process, renal impairment, or the concomitant presence of other medical conditions. Thus, the authors concluded that long-term sustained freedom from steroids may not be possible in all patients under Tac secondary to these conditions. Another multicenter, 1-year, comparative, double-blind, placebo-controlled study evaluating the efficacy and safety of an immunosuppressive regimen with steroid withdrawal at day 14 revealed a higher incidence of acute rejection, only balanced by a trend of a lower need for antidiabetic treatment [40]. The previous study suggested that steroid withdrawal or avoidance may not always be safe and needed. Steroid reintroduction may be necessary for late rejection episodes, recurrent autoimmune disease, or renal impairment due to Tac. As mentioned above, there are three categories of individuals in whom the long-term adverse effects of steroids after liver transplantation are particularly detrimental. First, most steroid-induced side effects occurred in children, like what was encountered in adults. Of note, growth retardation and Cushingoid features are of concern in the pediatric transplant recipients. Second, those patients with cardiovascular risk factors of hyperlipidemia, hypertension, or diabetes certainly have a contraindication to long-term steroid therapy. Last but not least, it could be supposed that rapid steroid tapering or being steroid free will serve as one of the most important determinants for slowing down the progression to tumor relapse.
For years corticosteroid induction has been the traditional standard immunosuppressive modality for OLT. Recently, induction therapy with antibodies has been increasingly used without widespread acceptance. The underlying mechanism of induction therapy is to inhibit thymus-derived lymphocyte activation through T cell pool depletion with either monoclonal antibodies or polyclonal antibodies (RATG), or to block specific IL-2 receptors (basiliximab or daclizumab), which may lead to reduction of the incidence of acute rejection and act as steroid-sparing minimization alternatives [19]. Mangus et al. [20] retrospectively analyzed data obtained from a single-center research database ranging from 2001 to 2008 comparing transplant outcomes and complications. The authors concluded that RATG-based induction immunosuppression could be safely used in adult OLT recipients with excellent survival, low rejection rates, and a comparably acceptable incidence of side effects [20]. Experts from the University of Tokyo in Japan conducted an observational study to evaluate the efficacy and safety of basiliximab as rescue therapy for the treatment of acute cellular rejection [41]. In contrast to 11 patients who received steroid therapy for acute cellular rejection, there were no significant immediate adverse effects in the basiliximab group which underwent liver transplantation for HCV cirrhosis [41]. In addition, recent studies have shown that immunosuppression with low-dose daclizumab and delayed initiation of Tac had significant benefits in preserving renal function after OLT [42]. However, the application of the induction therapy with biologic agents carrying elevated risks of over-immunosuppression, CMV viremia, posttransplant lymphoproliferative disease, as well as HCV recurrence is still controversial [43]. In our present study, we have demonstrated comparable patient and graft survival with significantly lower rates of HCV recurrence, diabetes, bacterial infection, and CMV infection as compared to no induction intervention.
Conclusions
In conclusion, the present investigation systematically reviewed the recent 17 prospective randomized controlled clinical trials concerning the application of steroid minimization in the Tac-based immunosuppressive regimen for liver transplant recipients, and a meta-analysis was performed to reveal the efficacy and safety of early steroid withdrawal or complete avoidance. Furthermore, adverse events potentially related to steroids were less frequently observed with the use of antibody agents for induction therapy while low-dose Tac could be maintained.
Abbreviations
- AIH:
-
Autoimmune hepatitis
- CI:
-
Confidence intervals
- CMV:
-
Cytomegalovirus
- EBV:
-
Epstein–Barr virus
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- MELD:
-
Model for end-stage liver disease
- MMF:
-
Mycophenolate mofetil
- OLT:
-
Orthotopic liver transplantation
- PBC:
-
Primary biliary cirrhosis
- PSC:
-
Primary sclerosing cholangitis
- RATG:
-
Rabbit antithymocyte globulin
- RCT:
-
Randomized controlled trials
- RR:
-
Risk ratios
- Tac:
-
Tacrolimus
References
Merion RM. Current status and future of liver transplantation. Semin Liver Dis 2010;30:411–421
Sánchez-Fueyo A, Strom TB. Immunologic basis of graft rejection and tolerance following transplantation of liver or other solid organs. Gastroenterology 2011;140:51–64
Levitsky J. Operational tolerance: past lessons and future prospects. Liver Transpl 2011;17:222–232
Fändrich F. Tolerance in clinical transplantation: progress, challenge or just a dream? Langenbecks Arch Surg 2011;396:475–87
Salcedo M, Vaquero J, Bañares R, Rodríguez-Mahou M, Alvarez E, Vicario JL, et al. Response to steroids in de novo autoimmune hepatitis after liver transplantation. Hepatology 2002;35:349–356
Gruttadauria S, di Francesco F, Pagano D, Vizzini G, Cintorino D, Spada M, et al. Complications in immunosuppressive therapy of liver transplant recipients. J Surg Res 2011;168:e137–e142
Bhat I, Mukherjee S. Hepatitis C recurrence after liver transplantation. Panminerva Med 2009;51:235–247
Ciesek S, Steinmann E, Iken M, Ott M, Helfritz FA, Wappler I, et al. Glucocorticosteroids increase cell entry by hepatitis C virus. Gastroenterology 2010;138:1875–1884
Chen ZS, He F, Zeng FJ, Jiang JP, Du DF, Liu B. Early steroid withdrawal after liver transplantation for hepatocellular carcinoma. World J Gastroenterol 2007;13:5273–5276
Park JW, Lee KW, Kim SJ, Choi SH, Heo JS, Kwon CH, et al. Outcome of patients with recurrent hepatocellular carcinoma in liver transplantation. Transplant Proc 2006;38:2121–2122
Pirenne J, Aerts R, Koshiba T, Van Gelder F, Roskams T, Schetz M, et al. Steroid-free immunosuppression during and after liver transplantation—a 3-yr follow-up report. Clin Transplant 2003;17:177–182
Ringe B, Braun F, Schütz E, Füzesi L, Lorf T, Canelo R, et al. A novel management strategy of steroid-free immunosuppression after liver transplantation: efficacy and safety of tacrolimus and mycophenolate mofetil. Transplantation 2001;71:508–515
Diem HV, Sokal EM, Janssen M, Otte JB, Reding R. Steroid withdrawal after pediatric liver transplantation: a long-term follow-up study in 109 recipients. Transplantation 2003;75:1664–1670
Reding R, Gras J, Sokal E, Otte JB, Davies HF. Steroid-free liver transplantation in children. Lancet 2003;362:2068–2070
Samonakis DN, Mela M, Quaglia A, Triantos CK, Thalheimer U, Leandro G, et al. Rejection rates in a randomised trial of tacrolimus monotherapy versus triple therapy in liver transplant recipients with hepatitis C virus cirrhosis. Transpl Infect Dis 2006;8:3–12
Moench C, Barreiros AP, Schuchmann M, Bittinger F, Thiesen J, Hommel G, et al. Tacrolimus monotherapy without steroids after liver transplantation—a prospective randomized double—blinded placebo-controlled trial. Am J Transplant 2007;7:1616–1623
Reggiani P, Arru M, Regazzi M, Gatti S, Molinaro MD, Caccamo L, et al. A steroid-free tacrolimus and low-dose mycophenolate mofetil primary immunosuppression does not prevent early acute rejection after liver transplantation. Transplant Proc 2005;37:1697–1699
Foroncewicz B, Mucha K, Ryszkowska E, Ciszek M, Ziółkowski J, Porowski D, et al. Safety and Efficacy of steroid-free immunosuppression with tacrolimus and daclizumab in liver transplant recipients: 6-year follow-up in a single center. Transplant Proc 2009;41:3103–3106
Figueras J, Prieto M, Bernardos A, Rimola A, Suárez F, de Urbina JO, et al. Daclizumab induction and maintenance steroid-free immunosuppression with mycophenolate mofetil and tacrolimus to prevent acute rejection of hepatic allografts. Transpl Int 2006;19:641–648
Mangus RS, Fridell JA, Vianna RM, Kwo PY, Chen J, Tector AJ. Immunosuppression induction with rabbit antithymocyte globulin (rATG) ± rituximab in 1000 liver transplants with long-term follow-up. Liver Transpl 2012;18:786–795
Sato K, Sekiguchi S, Kawagishi N, Akamatsu Y, Ishida K, Fukushima D, et al. Unique histopathological features of graft biopsies with liver function abnormalities in living donor liver transplant patients receiving basiliximab induction therapy. Clin Transplant 2011;25:61–68
Moher D, Liberati A, Tetzlaff J, Altman DG, Group PRISMA. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097
Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.1. The Cochrane Collaboration; 2008. www.cochrane-handbook.org. Accessed 2 July 2009
Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. http://www.cochrane-handbook.org. Accessed 1 July 2012
Langrehr JM, Neumann UP, Lang M, Müller AR, Jonas S, Settmacher U, et al. First results from a prospective randomized trial comparing steroid-free induction therapy with tacrolimus and MMF versus tacrolimus and steroids in patients after liver transplantation for HCV. Transplant Proc 2002;34:1565–1566
Pelletier SJ, Vanderwall K, Debroy MA, Englesbe MJ, Sung RS, Magee JC, et al. Preliminary analysis of early outcomes of a prospective, randomized trial of complete steroid avoidance in liver transplantation. Transplant Proc 2005;37:1214–1216
Margarit C, Bilbao I, Castells L, Lopez I, Pou L, Allende E, et al. A prospective randomized trial comparing tacrolimus and steroids with tacrolimus monotherapy in liver transplantation: the impact on recurrence of hepatitis C. Transpl Int 2005;18:1336–1345
Junge G, Neuhaus R, Schewior L, Klupp J, Guckelberger O, Langrehr JM, et al. Withdrawal of steroids: a randomized prospective study of prednisone and tacrolimus versus mycophenolate mofetil and tacrolimus in liver transplant recipients with autoimmune hepatitis. Transplant Proc 2005;37:1695–1696
Vivarelli M, Burra P, La Barba G, Canova D, Senzolo M, Cucchetti A, et al. Influence of steroids on HCV recurrence after liver transplantation: a prospective study. J Hepatol 2007;47:793–798
Manousou P, Samonakis D, Cholongitas E, Patch D, O’Beirne J, Dhillon AP, et al. Outcome of recurrent hepatitis C virus after liver transplantation in a randomized trial of tacrolimus monotherapy versus triple therapy. Liver Transpl 2009;15:1783–1791
Weiler N, Thrun I, Hoppe-Lotichius M, Zimmermann T, Kraemer I, Otto G. Early steroid-free immunosuppression with FK506 after liver transplantation: long-term results of a prospectively randomized double-blinded trial. Transplantation 2010;90:1562–1566
Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE Jr. Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation 2003;75:1396–1399
Boillot O, Mayer DA, Boudjema K, Salizzoni M, Gridelli B, Filipponi F, et al. Corticosteroid-free immunosuppression with tacrolimus following induction with daclizumab: a large randomized clinical study. Liver Transpl 2005;11:61–67
Spada M, Petz W, Bertani A, Riva S, Sonzogni A, Giovannelli M, et al. Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression. Am J Transplant 2006;6:1913–1921
Humar A, Crotteau S, Gruessner A, Kandaswamy R, Gruessner R, Payne W, et al. Steroid minimization in liver transplant recipients: impact on hepatitis C recurrence and post-transplant diabetes. Clin Transplant 2007;21:526–531
Kato T, Gaynor JJ, Yoshida H, Montalvano M, Takahashi H, Pyrsopoulos N, et al. Randomized trial of steroid-free induction versus corticosteroid maintenance among orthotopic liver transplant recipients with hepatitis C virus: impact on hepatic fibrosis progression at one year. Transplantation 2007;84:829–835
Gras JM, Gerkens S, Beguin C, Janssen M, Smets F, Otte JB, et al. Steroid-free, tacrolimus-basiliximab immunosuppression in pediatric liver transplantation: clinical and pharmacoeconomic study in 50 children. Liver Transpl 2008;14:469–477
Klintmalm GB, Davis GL, Teperman L, Netto GJ, Washburn K, Rudich SM, et al. A randomized, multicenter study comparing steroid-free immunosuppression and standard immunosuppression for liver transplant recipients with chronic hepatitis C. Liver Transpl 2011;17:1394–1403
Jain A, Kashyap R, Marsh W, Rohal S, Khanna A, Fung JJ. Reasons for long-term use of steroid in primary adult liver transplantation under tacrolimus. Transplantation 2001;71:1102–1106
Pageaux GP, Calmus Y, Boillot O, Ducerf C, Vanlemmens C, Boudjema K, et al. Steroid withdrawal at day 14 after liver transplantation: a double-blind, placebo-controlled study. Liver Transpl 2004;10:1454–60
Togashi J, Sugawara Y, Tamura S, Kaneko J, Yamashiki N, Aoki T, et al. Basiliximab as therapy for acute rejection after liver transplantation for hepatitis C virus cirrhosis. Biosci Trends 2011;5:57–60
Post M, Raszeja-Wyszomirska J, Jarosz K, Lubikowski J, Wasilewicz M, Mydłowska M, et al. Immunosuppression with low-dose daclizumab in liver transplant recipients with impaired kidney function: a single-center experience. Transplant Proc 2009;41:3107–3109
Ramirez CB, Marino IR. The role of basiliximab induction therapy in organ transplantation. Expert Opin Biol Ther 2007;7:137–148
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81170417, 81300337), Natural Science Foundation of Jiangsu Province (BK2012081), Natural Science Foundation for Health Department of Jiangsu Province (Q201307), China Postdoctoral Science Foundation (2011M500904), Jiangsu Planned Projects for Postdoctoral Research Funds (1102149C), Nan**g Medical Science and Technique Development Foundation and Medjaden Young Scientists Fund (MJR20130001).
Compliance with ethical requirements and Conflict of interest
The authors declare that they have no ethical conflicts. **yang Gu, **ngyu Wu, Lu Lei, Zhang Shu, Jianling Bai, Jun Wang, Jun Li, and Yitao Ding declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
**yang Gu, **ngyu Wu, and Lei Lu have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 5
Representative funnel plot of 1-year patient survival rate in Sect. I (TIFF 3051 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Gu, J., Wu, X., Lu, L. et al. Role of steroid minimization in the tacrolimus-based immunosuppressive regimen for liver transplant recipients: a systematic review and meta-analysis of prospective randomized controlled trials. Hepatol Int 8, 198–215 (2014). https://doi.org/10.1007/s12072-014-9523-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-014-9523-y