Abstract
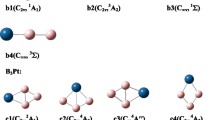
The potential energy surface (PES) has been explored for BSinGe4−n+ (n = 0−2) systems using density functional theory (DFT). The global minima (1a, 1b, and 1c) of the considered systems contain a planar tetracoordinate boron (ptB) center. The neutral states of the systems do not have a ptB in the global minimum structures. The designed BGe4+, BSiGe3+, and BSi2Ge2+ systems have 18 valence electrons. The CCSD(T)/aug-cc-pVTZ level of theory has been applied to compute the relative energies of the low-lying isomers with respect to the global minima. The dynamical stability of BSinGe4−n+ (n = 0−2) systems is confirmed from the atom-centered density matrix propagation (ADMP) simulation over 20 ps of time at temperatures of 300 K and 500 K. The natural charge computations show that the charges on the ptB are highly negative, indicating strong σ-acceptance from the peripheral atoms. The 1a, 1b, and 1c structures of BGe4+, BSiGe3+, and BSi2Ge2+ systems, respectively, have σ/π-dual aromaticity as predicted from the nucleus-independent chemical shift (NICS) values.
Graphical abstract
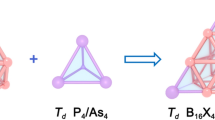
Density functional theory (DFT) based computation predicts the presence of a planar tetracoordinate boron (ptB) in the global minimum energy structures of BSinGe4−n+ (n = 0−2) systems. The systems are kinetically stable and show σ- and π- electronic delocalization.












Similar content being viewed by others
References
van’t Hoff J H 1874 A suggestion looking to the extension into space of the structural formulas at present used in chemistry, and a note upon the relation between the optical activity and the chemical constitution of organic compounds Arch. Neerl. Sci. Exactes Nat. 9 445
Le-Bel J A 1874 On the relations which exist between the atomic formulas of organic compounds and the rotatory power of their solutions Bull. Soc. Chim. Fr. 22 337
Monkhorst H J 1968 Activation energy for interconversion of enantiomers containing an asymmetric carbon atom without breaking bonds Chem. Commun. 11 1111
Hoffmann R, Alder R W and Wilcox C F 1970 Planar tetracoordinate carbon J. Am. Chem. Soc. 92 4992
Collins J B, Dill J D, Jemmis E D, Apeloig Y, von Ragué Schleyer P, Seeger R and Pople J A 1976 Stabilization of planar tetracoordinate carbon J. Am. Chem. Soc. 98 5419
Merino G, Méndez-Rojas M A, Beltrán H I, Corminboeuf C, Heine T and Vela A 2004 Theoretical analysis of the smallest carbon cluster containing a planar tetracoordinate carbon J. Am. Chem. Soc. 126 16160
Yang L M, Ganz E, Chen Z, Wang Z X and von Ragué Schleyer P 2015 Four decades of the chemistry of planar hypercoordinate compounds Angew. Chem. Int. Ed. 54 9468
Cui Z H, Contreras M, Ding Y H and Merino G 2011 Planar tetracoordinate carbon versus planar tetracoordinate boron: The case of CB4 and its cation J. Am. Chem. Soc. 133 13228
Cui Z H, Ding Y H, Cabellos J L, Osorio E, Islas R, Restrepo A and Merino G 2015 Planar tetracoordinate carbons with a double bond in CAl3E clusters Phys. Chem. Chem. Phys. 17 8769
Das P, Pan S and Chattaraj P K 2023 Planar hypercoordinate carbon In: Atomic Clusters with Unusual Structure, Bonding and Reactivity (Elsevier) p. 357
Das P and Chattaraj P K 2021 In silico studies on selected neutral molecules, CGa2Ge2, CAlGaGe2, and CSiGa2Ge containing planar tetracoordinate carbon Atoms 9 65
Das P and Chattaraj P K 2022 CSiGaAl2−/0 and CGeGaAl2−/0 having planar tetracoordinate carbon atoms in their global minimum energy structures J. Comput. Chem. 43 894
Das P, Khatun M, Anoop A and Chattaraj P K 2022 CSinGe4−n2+ (n = 1–3): prospective systems containing planar tetracoordinate carbon (ptC) Phys. Chem. Chem. Phys. 24 16701
Li X, Wang L S, Boldyrev A I and Simons J 1999 Tetracoordinated planar carbon in the Al4C− anion. A combined photoelectron spectroscopy and ab initio study J. Am. Chem. Soc. 121 6033
Boldyrev A I and Simons J 1998 Tetracoordinated planar carbon in pentaatomic molecules J. Am. Chem. Soc. 120 7967
Röttger D and Erker G 1997 Compounds containing planar-tetracoordinate carbon Angew. Chem. Int. Ed. Engl. 36 812
Erker G 1992 Planar-tetracoordinate carbon: Making stable anti-van’t Hoff/Le Bel compounds Comment. Inorg. Chem. 13 111
Keese R 2006 Carbon flatland: Planar tetracoordinate carbon and fenestranes Chem. Rev. 106 4787
Xu J, Zhang X, Yu S, Ding Y H and Bowen K H 2017 Identifying the hydrogenated planar tetracoordinate carbon: A combined experimental and theoretical study of CAl4H and CAl4H− J. Phys. Chem. Lett. 8 2263
Li X, Zhang H F, Wang L S, Geske G and Boldyrev A 2000 Pentaatomic tetracoordinate planar carbon, [CAl4]2−: A new structural unit and its salt complexes Angew. Chem. Int. Ed. 39 3630
Pei Y, An W, Ito K, von Ragué Schleyer P and Zeng X C 2008 Planar pentacoordinate carbon in CAl5+: A global minimum J. Am. Chem. Soc. 130 10394
Vassilev-Galindo V, Pan S, Donald J K and Merino G 2018 Planar pentacoordinate carbons Nat. Chem. Rev. 2 0114
Pan S, Cabellos J L, Orozco M, Merino G, Zhao L and Chattaraj P K 2018 Planar pentacoordinate carbon in CGa5+ derivatives Phys. Chem. Chem. Phys. 20 12350
Exner K and von Ragué Schleyer P 2000 Planar hexacoordinate carbon: A viable possibility Science 290 1937
Averkiev B B, Zubarev D Y, Wang L M, Huang W, Wang L S and Boldyrev A I 2008 Carbon avoids hypercoordination in CB6−, CB62−, and C2B5− planar carbon-boron clusters J. Am. Chem. Soc. 130 9248
Wu Y B, Duan Y, Lu G, Lu H G, Yang P, Schleyer P and v R, Merino G, Islas R and Wang Z X, 2012 D3h CN3Be3+ and CO3Li3+: Viable planar hexacoordinate carbon prototypes Phys. Chem. Chem. Phys. 14 14760
Parra L L, Diego L, Yañez O, Inostroza D, Barroso J, Vásquez-Espinal A, et al. 2021 Planar hexacoordinate carbons: Half covalent, half ionic Angew. Chem. Int. Ed. 60 8700
Minyaev R M, Gribanova T N, Starikov A G and Minkin V I 2002 Heptacoordinated carbon and nitrogen in a planar boron ring Dokl. Chem. 382 41
Wang L M, Huang W, Averkiev B B, Boldyrev A I and Wang L S 2007 CB7−: Experimental and theoretical evidence against hypercoordinate planar carbon Angew. Chem. Int. Ed. 46 4550
Castillo-Toraya G, Orozco-Ic M, Dzib E, Zarate X, Ortíz-Chi F, Cui Z, et al. 2021 Planar tetracoordinate fluorine atoms Chem. Sci. 12 6699
Chen C, Wang M, Feng L Y, Zhao L Q, Guo J C, Zhai H J, et al. 2022 Bare and ligand protected planar hexacoordinate silicon in SiSb3M3+ (M = Ca, Sr, Ba) clusters Chem. Sci. 13 8045
Wang M, Chen C, Pan S and Cui Z 2021 Planar hexacoordinate gallium Chem. Sci. 12 15067
Kalita A J, Sarmah K, Yashmin F, Borah R R, Baruah I, Deka R P and Guha A K 2022 σ-Aromaticity in planar pentacoordinate aluminium and gallium clusters Sci. Rep. 12 10041
Feng W, Zhu C, Liu X, Zhang M, Geng Y, Zhao L and Su Z 2020 A BPt4S4 cluster: A planar tetracoordinate boron system with three charges all at their global energy minima New J. Chem. 44 767
Li S D, Miao C Q and Ren G M 2004 D5h Cu5H5X: Pentagonal hydrocopper Cu5H5 containing pentacoordinate planar nonmetal centers (X = B, C, N, O) Eur. J. Inorg. Chem. 2004 2232
Yu H L, Sang R L and Wu Y Y 2009 Structure and aromaticity of B6H5+ cation: A novel borhydride system containing planar pentacoordinated boron J. Phys. Chem. A 113 3382
Khatun M, Roy S, Giri S, Ch S S R, Anoop A and Thimmakondu V S 2021 BAl4Mg−/0/+: Global minima with a planar tetracoordinate or hypercoordinate boron Atom Atoms 9 89
Gribanova T N, Minyaev R M and Minkin V I 2001 Planar hexacoordinated boron in organoboron compounds: an ab initio study Mendeleev Commun. 11 169
Das P, Patra S G and Chattaraj P K 2022 CB6Al0/+: Planar hexacoordinate boron (phB) in the global minimum structure Phys. Chem. Chem. Phys. 24 22634
Zhai H J, Alexandrova A N, Birch K A, Boldyrev A I and Wang L S 2003 Hepta- and octacoordinate boron in molecular wheels of eight- and nine-atom boron clusters: Observation and confirmation Angew. Chem., Int. Ed. 42 6004
Zhao T, Wang Q and Jena P 2016 Cluster-inspired design of high-capacity anode for Li-ion batteries ACS Energy Lett. 1 202
Dai J, Wu X, Yang J and Zeng X C 2014 AlxC monolayer sheets: two-dimensional networks with planar tetracoordinate carbon and potential applications as donor material in solar cell J. Phys. Chem. Lett. 5 2058
Zhang J and Dolg M 2015 ABCluster: the artificial bee colony algorithm for cluster global optimization Phys. Chem. Chem. Phys. 17 24173
Zhang J and Dolg M 2016 Global optimization of clusters of rigid molecules using the artificial bee colony algorithm Phys. Chem. Chem. Phys. 18 3003
Karaboga D 2005 An Idea Based on Honey Bee Swarm for Numerical Optimization, Technical Report-TR06, Erciyes University, Engineering Faculty, Computer Engineering Department
Sarkar K and Bhattacharyya S P 2017 Soft Computing in Chemical and Physical Sciences: A Shift in Computing Paradigm 1st edn. (Boca Raton: CRC Press)
Adamo C and Barone V 1999 Toward reliable density functional methods without adjustable parameters: The PBE0 model J. Chem. Phys. 110 6158
Grimme S, Antony J, Ehrlich S and Krieg H 2010 A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu J. Chem. Phys. 132 154104
Weigend F and Ahlrichs R 2005 Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy Phys. Chem. Chem. Phys. 7 3297
Scuseria G E and Schaefer H F 1989 Is coupled cluster singles and doubles (CCSD) more computationally intensive than quadratic configuration interaction (QCISD) J. Chem. Phys. 90 3700
Weigend F 2006 Accurate coulomb-fitting basis sets for H to Rn Phys. Chem. Chem. Phys. 8 1057
Frisch M J, Trucks G W, Schlegel H B, Scuseria G E, Robb M A, Cheeseman J R, et al. 2016 Gaussian 16, Revision B.01 (Gaussian Inc: Wallingford, CT)
Schlegel H B, Millam J M, Iyengar S S, Voth G A, Daniels A D, Scuseria G E and Frisch M J 2001 Ab initio molecular dynamics: Propagating the density matrix with Gaussian orbitals J. Chem. Phys. 114 9758
Iyengar S S, Schlegel H B, Millam J M, Voth G A, Scuseria G E and Frisch M J 2001 Ab initio molecular dynamics: Propagating the density matrix with Gaussian orbitals. II. Generalizations based on mass-weighting, idempotency, energy conservation and choice of initial conditions J. Chem. Phys. 115 10291
Schlegel H B, Iyengar S S, Li X, Millam J M, Voth G A, Scuseria G E and Frisch M J 2002 Ab initio molecular dynamics: Propagating the density matrix with Gaussian orbitals. III. Comparison with Born-Oppenheimer dynamics J. Chem. Phys. 117 8694
Das P, Saha R and Chattaraj P K 2020 Encapsulation of Mg2 inside a C60 cage forms an electride J. Comput. Chem. 41 1645
Reed A E, Curtiss L A and Weinhold F 1988 Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint Chem. Rev. 88 899
Møller C and Plesset M S 1934 Note on an approximation treatment for many electron systems Phys. Rev. 46 618
Cremer D 2011 Møller-Plesset perturbation theory: from small molecule methods to methods for thousands of atoms Wiley Interdiscip Rev.: Comput. Mol. Sci. 1 509
Dunning T H Jr 1989 Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen J. Chem. Phys. 90 1007
Kendall R A, Dunning T H Jr and Harrison R J 1992 Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions J. Chem. Phys. 96 6796
Woon D E and Dunning T H Jr 1993 Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon J. Chem. Phys. 98 1358
Bader R F W 1990 Atoms in Molecules. In A Quantum Theory (Oxford University Press: Oxford, UK)
Lu T and Chen F 2012 Multiwfn: A multifunctional wavefunction analyzer J. Comput. Chem. 33 580
Zubarev D Y and Boldyrev A I 2008 Develo** paradigms of chemical bonding: adaptive natural density partitioning Phys. Chem. Chem. Phys. 10 5207
Zubarev D Y and Boldyrev A I 2008 Revealing intuitively assessable chemical bonding patterns in organic aromatic molecules via adaptive natural density partitioning J. Org. Chem. 73 9251
von Ragué Schleyer P, Jiao H, Hommes N v E, Malkin V G and Malkina O L 1997 An evaluation of the aromaticity of inorganic rings: refined evidence from magnetic properties J. Am. Chem. Soc. 119 12669
von Ragué Schleyer P, Maerker C, Dransfeld A, Jiao H and van Eikema Hommes N J R 1996 Nucleus-Independent chemical shifts: A simple and efficient aromaticity probe J. Am. Chem. Soc. 118 6317
Krygowski T M and Cyranski M 2001 Structural aspects of aromaticity Chem. Rev. 101 1385
Das P and Chattaraj P K 2020 Electride characteristics of some binuclear sandwich complexes of alkaline earth metals, M2(η5-L)2 (M = Be, Mg; L = C5H5–, N5–, P5–, As5–) J. Phys. Chem. A 124 9801
Das P and Chattaraj P K 2021 Substituent effects on electride characteristics of Mg2(η5-C5H5)2: a theoretical study J. Phys. Chem. A 125 6207
Acknowledgements
PKC would like to thank DST, New Delhi, India, for the J. C. Bose National Fellowship, grant number SR/S2/JCB-09/2009. PD thanks UGC, New Delhi, India, for the Research Fellowship. A part of the computation was carried out by the resources of the Supercomputing facility at the Indian Institute of Technology Kharagpur, established under the National Supercomputing Mission (NSM), Government of India and supported by the Centre for Development of Advanced Computing (CDAC), Pune.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interest.
Additional information
Dedicated to Professor S. P. Bhattacharyya on the occasion of his 75th birthday
Special Issue on Interplay of Structure and Dynamics in Reaction Pathways, Chemical Reactivity and Biological Systems
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Das, P., Chattaraj, P.K. BSinGe4−n+ (n = 0−2): prospective systems containing planar tetracoordinate boron (ptB). J Chem Sci 135, 1 (2023). https://doi.org/10.1007/s12039-022-02121-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-022-02121-6