Abstract
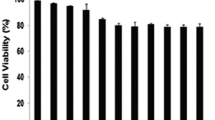
According to the method used in our laboratory, our group synthesized (DIPP-Trp)2-Lys-OCH3. It inhibited the proliferation of K562 and HeLa cells in a dose-and time-dependent manner with an IC50 of 15.12 and 42.23 µM, respectively. (DIPP-Trp)2-Lys-OCH3 induced a dose-dependent increase of the G2/M cell population in K562 cells, and S cell population in HeLa cells; the sub-G0 population increased dramatically in both cell lines as seen by PI staining experiments using a FACS Calibur Flow cytometer (BeckmanCoulter, USA). Phosphatidylserine could significantly translocate to the surface of the membrane in (DIPP-Trp)2-Lys-OCH3-treated K562 and HeLa cells. The increase of an early apoptotic population was observed in a dose-dependent manner by both annexin-FITC and PI staining. It was concluded that (DIPP-Trp)2-Lys-OCH3 not only induced cells to enter into apoptosis, but also affected the progress of the cell cycle. It may have arrested the K562 and HeLa cells in the G2/M, S phases, respectively. The apoptotic pathway was pulsed at this point, resulting in the treated cells entering into programmed cell death. (DIPP-Trp)2-Lys-OCH3 is a potential anticancer drug that intervenes in the signalling pathway.
Similar content being viewed by others
Abbreviations
- Apaf-1:
-
apoptotic protease activating factor-1
- (DIPP-Trp)2-Lys-OCH3 :
-
(di-isopropyloxyphoryl-Trp)2-Lys-OCH3
- FBS:
-
foetal bovine serum
- IC50 :
-
inhibitory concentration 50%
- IR%:
-
inhibition rate
- MTT:
-
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- PI:
-
propidium iodide
- PS:
-
phosphatidylserine
References
Bertenshaw S R, Rogers R S, Stern M K, Norman B H, Moore W M, Jerome G M, Branson L M, McDonald J F, McMahon E G and Palomo M A 1993 Phosphorus-containing inhibitors of endothelin converting enzyme: effects of the electronic nature of phosphorus on inhibitor potency; J. Med. Chem. 36 173–176
Cai Y M, Gao X, Huang X T, Wang T J and Zhao Y F 2006 Synthesis, spectral characterization and biological activities of phosphoryl amino acid esters; Chinese J. Org. Chem. 26 1677–1681
Cheng C M, Liu X H, Li Y M, Ma Y, Tan B, Wan R and Zhao Y F 2004 N-phosphoryl amino acids and biomolecular origins; Origins Life Evol. Biosphere 34 455–464
Hanahan D and Weinberg R A 2000 The hallmarks of cancer; Cell 100 57–70
Jacobson M D, Weil M and Raff M C 1997 Programmed cell death in animal development; Cell 88 347–354
Ji G J, Xue C B, Zeng J N, Li L P, Chai W G and Zhao Y F 1988 Synthesis of N-(diisoproxy phosphoryl) amino acids and peptide; Synthesis 6 444–448
Johnstone R W, Ruefli A A and Lowe S W 2002 Apoptosis: a link between cancer genetics and chemotherapy; Cell 108 153–164
Jordan M A, Wendell K, Gardiner S, Derry W B, Copp H and Wilson L 1996 Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death; Cancer Res. 56 816–825
Kastan M B, Canman C E and Leonard C J 1995 P53, cell cycle control and apoptosis: implications for cancer; Cancer Metastasis Rev. 14 3–15
Kidd V J 1998 Proteolytic activities that mediate apoptosis; Annu. Rev. Physiol. 60 533–573
Kornblau S M 1998 The role of apoptosis in the pathogenesis, prognosis, and therapy of hematologic malignancies; Leukemia 12 S41–S46
Kortylewicz Z P 1990 Phosphoramidate peptide inhibition of human skin fibroblast collagenase; J. Med. Chem. 33 263–273
Li P, Nijhawan D, Budihardjo I, Srinivasula S M, Ahmad M, Alnemri E S and Wang X D 1997 Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade; Cell 91 479–489
Mosmann T 1983 Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytoxicity assays; J. Immunol. Methods 65 55–63
Nagata S 1997 Apoptosis by death factor; Cell 88 355–365
Nayfield S G, Karp J E, Ford L G, Dorr F A and Kramer B S 1991 Potential role of tamoxifen in prevention of breast cancer; J. Natl. Cancer Inst. 83 1450–1459
Niu Y L, Du W, Jiang Y Y, Cao S L and Zhao Y F 2001 Apoptosis of HCT-15 cell lines induced by a kind of N-phosphoryl branched dipeptide; J. Tsinghua Univ. 7 101–103 (S1)
Reeves J P 1979 Accumulation of amino acids by lysosomes incubated with amino acid methyl esters; J. Biol. Chem. 254 8914–8921
Sausville E A, Elsayed Y, Monga M and Kim G 2003 Signal transduction-directed cancer treatments; Annu. Rev. Pharmacol. Toxicol. 43 199–231
Schulte-Hermann R, Bursch W, Low-Baselli A, Wagner A and Grasl-Kraupp B 1997 Apoptosis in the liver and its role in hepatocarcinogenesis; Cell. Biol. Toxicol. 13 339–348
Smets A 1994 Programmed cell death (apoptosis) and response to anti-cancer drugs; Anticancer Drugs 5 3–9
Thiele D L 1992 Apoptosis is induced in cells with cytolytic potential by L-leucyl-L-leucine methyl ester; J. Immunol. 148 3950–3957
Thiele D L and Lipsky P E 1985 Modulation of human natural killer cell function by L-leucine methyl ester: monocyte-dependent depletion from human peripheral blood mononuclear cells; J. Immunol. 134 786–793
Thompson C B 1995 Apoptosis in the pathogenesis and treatment of disease; Science 267 1456–1462
Van Engeland M, Nieland L J W, Ramaekers F C S, Schutte B and Reutelingsperger C P M 1998 Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure; Cytometry 31 1–9
Yang J, Jiang Y Y, Liu F and Zhao Y F 2006 Mitochondria-regulated death pathway mediates (DIPP-L-Leu)(2)-L-LysOCH(3)-induced K562 cells apoptosis; Protein Peptide Lett. 13 129–134
Zhao Y F, Ju Y, Li Y M, Wang Q, Yin Y W and Tan B 1995 Self-activation of N-phosphoamino acids and N-phosphodipeptide in oligopeptide formation; Int. J. Peptide Protein Res. 45 514–518
Zhao Y F, Zhang J C, Cao S X, Xu J, Rong C L and Qu L B 2004 Synthesis of N-(diisopropyloxyphosphoryl) amino acids; Chinese J. Org. Chem. 24 609–615
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, F., Liu, SY., Xu, P. et al. Apoptosis induced by (di-isopropyloxyphoryl-Trp)2-Lys-OCH3 in K562 and HeLa cells. J Biosci 33, 55–62 (2008). https://doi.org/10.1007/s12038-008-0001-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12038-008-0001-3