Abstract
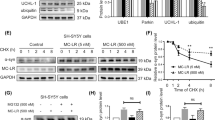
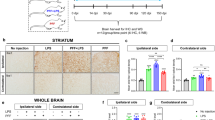
The aim of this study is to investigate the role and mechanism of microglial NOX2 activation in minimally toxic dose of LPS and Syn-elicited synergistic dopaminergic neurodegeneration. NOX2+/+ and NOX2−/− mice and multiple primary cultures were treated with LPS and/or Syn in vivo and in vitro. Neuronal function and morphology were evaluated by uptake of related neurotransmitter and immunostaining with specific antibody. Levels of superoxide, intracellular reactive oxygen species, mRNA and protein of relevant molecules, and dopamine were detected. LPS and Syn synergistically induce selective and progressive dopaminergic neurodegeneration. Microglia are functionally and morphologically activated, contributing to synergistic dopaminergic neurotoxicity elicited by LPS and Syn. NOX2−/− mice are more resistant to synergistic neurotoxicity than NOX2+/+mice in vivo and in vitro, and NOX2 inhibitor protects against synergistic neurotoxicity through decreasing microglial superoxide production, illustrating a critical role of microglial NOX2. Microglial NOX2 is activated by LPS and Syn as mRNA and protein levels of NOX2 subunits P47and gp91 are enhanced. Molecules relevant to microglial NOX2 activation include PKC-σ, P38, ERK1/2, JNK, and NF-КBP50 as their mRNA and protein levels are elevated after treatment with LPS and Syn. Combination of exogenous and endogenous environmental factors with minimally toxic dose synergistically propagates dopaminergic neurodegeneration through activating microglial NOX2 and relevant signaling molecules, casting a new light for PD pathogenesis.








Similar content being viewed by others
References
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8(1):57–69. doi:10.1038/nrn2038
Lull ME, Block ML (2010) Microglial activation and chronic neurodegeneration. Neurotherapeutics 7(4):354–365. doi:10.1016/j.nurt.2010.05.014
Kannarkat GT, Boss JM, Tansey MG (2013) The role of innate and adaptive immunity in Parkinson’s disease. J Parkinsons Dis 3(4):493–514. doi:10.3233/JPD-130250
Levesque S, Taetzsch T, Lull ME, Johnson JA, McGraw C, Block ML (2013) The role of MAC1 in diesel exhaust particle-induced microglial activation and loss of dopaminergic neuron function. J Neurochem 125(5):756–765. doi:10.1111/jnc.12231
Mangano EN, Litteljohn D, So R, Nelson E, Peters S, Bethune C, Bobyn J, Hayley S (2012) Interferon-γ plays a role in paraquat-induced neurodegeneration involving oxidative and proinflammatory pathways. Neurobiol Aging 33(7):1411–1426. doi:10.1016/j.neurobiolaging.2011.02.016
Yuan YH, Sun JD, Wu MM, Hu JF, Peng SY, Chen NH (2013) Rotenone could activate microglia through NFκB associated pathway. Neurochem Res 38(8):1553–1560. doi:10.1007/s11064-013-1055-7
Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B (2002) Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem 81(6):1285–1297
Ling Z, Zhu Y, Tong CW, Snyder JA, Lipton JW, Carvey PM (2006) Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol 199(2):499–512. doi:10.1016/j.expneurol.2006.01.010
Jung BD, Shin EJ, Nguyen XK, ** CH, Bach JH, Park SJ et al (2010) Potentiation of methamphetamine neurotoxicity by intrastriatal lipopolysaccharide administration. Neurochem Int 56(2):229–244. doi:10.1016/j.neuint.2009.10.005
Zhang W, Phillips K, Wielgus AR, Liu J, Albertini A, Zucca FA, Faust R, Qian SY et al (2011) Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson’s disease. Neurotox Res 19(1):63–72. doi:10.1007/s12640-009-9140-z
Bernstein AI, Garrison SP, Zambetti GP, O’Malley KL (2011) 6-OHDA generated ROS induces DNA damage and p53- and PUMA-dependent cell death. Mol Neurodegener 6(1):2. doi:10.1186/1750-1326-6-2
Cho Y, Son HJ, Kim EM, Choi JH, Kim ST, Ji IJ, Choi DH, Joh TH et al (2009) Doxycycline is neuroprotective against nigral dopaminergic degeneration by a dual mechanism involving MMP-3. Neurotox Res 16(4):361–371. doi:10.1007/s12640-009-9078-1
Levesque S, Wilson B, Gregoria V, Thorpe LB, Dallas S, Polikov VS, Hong JS, Block ML (2010) Reactive microgliosis: extracellular micro-calpain and microglia-mediateddopaminergic neurotoxicity. Brain 133(Pt 3):808–821. doi:10.1093/brain/awp333
McGeer PL, Itagaki S, Boyes BE, McGeer EG (1988) Reactive microglia are positive for HLA-DR in the substantianigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38(8):1285–1291
Yamada T, McGeer PL, McGeer EG (1992) Lewybodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol 84(1):100–104
Kaźmierczak A, Adamczyk A, Benigna-Strosznajder J (2013) The role of extracellular α-synuclein in molecular mechanisms of cell death. Postepy Hig Med Dosw 67:1047–1457
Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W et al (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J 19(6):533–542. doi:10.1096/fj.04-2751com
Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B, Miller DS et al (2007) Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia 55(11):1178–1188. doi:10.1002/glia.20532
Liu B, Jiang JW, Wilson B, Du L, Yang SN, Wang JY, Wu GC, Cao XD et al (2000) Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J Pharmacol Exp Ther 295(1):125–132
Ali SF, Newport GD, Holson RR, Slikker W Jr, Bowyer JF (1994) Low environmental temperatures or pharmacologic agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 658(1–2):33–38
Infanger DW, Sharma RV, Davisson RL (2006) NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal 8(9–10):1583–1596. doi:10.1089/ars.2006.8.1583
Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55(5):453–462. doi:10.1002/glia.20467
McGeer PL, McGeer EG (2008) Glial reactions in Parkinson’s disease. Mov Disord 23(4):474–483. doi:10.1002/mds.21751
Ouchi Y, Yagi S, Yokokura M, Sakamoto M (2009) Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism. Relat Disord Suppl 3:S200–S204. doi:10.1016/S1353-8020(09)70814-4
Block ML (2008) NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci 9(Suppl 2):S8. doi:10.1186/1471-2202-9-S2-S8
Tammariello SP, Quinn MT, Estus S (2000) NADPH oxidase contributes directly to oxidative stress and apoptosis in nerve growth factor-deprived sympathetic neurons. J Neurosci 20(1):RC53
Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS (2004) NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem 279(2):1415–1421. doi:10.1074/jbc.M307657200
Sorce S, Krause KH (2009) NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal 11(10):2481–2504. doi:10.1089/ARS.2009.2578
Kozikowski AP, Nowak I, Petukhov PA, Etcheberrigaray R, Mohamed A, Tan M, Lewin N, Hennings H et al (2003) New amide-bearing benzolactam-based protein kinase C modulators induce enhanced secretion of the amyloid precursor protein metabolite sAPPalpha. J Med Chem 46(3):364–373. doi:10.1021/jm020350r
Miller RL, James-Kracke M, Sun GY, Sun AY (2009) Oxidative and inflammatory pathways in Parkinson’s disease. Neurochem Res 34(1):55–65. doi:10.1007/s11064-008-9656-2
Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, Cathcart MK (2005) Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. J Leukoc Biol 77(3):414–420. doi:10.1189/jlb.0504284
Bey EA, Xu B, Bhattacharjee A, Oldfield CM, Zhao X, Li Q, Subbulakshmi V, Feldman GM et al (2004) Protein kinase C delta is required for p47phox phosphorylation and translocation in activated human monocytes. J Immunol 173(9):5730–5738
Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH (2004) Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia 48(3):197–206. doi:10.1002/glia.20069
Murphy LO, Blenis J (2006) MAPK signal specificity: the right place at the right time. Trends Biochem Sci 31(5):268–275. doi:10.1016/j.tibs.2006.03.009
Bardwell L (2006) Mechanisms of MAPK signalling specificity. BiochemSoc Trans 34(Pt5):837–841. doi:10.1042/BST0340837
Flohe L, Brigelius-Flohe R, Saliou C, Traber MG, Packer L (1997) Redox regulation of NF-kappa B activation. Free RadicBiol Med 22(6):1115–1126. doi:10.1016/S0891-5849(96)00501-1
Yang S, Yang J, Yang Z, Chen P, Fraser A, Zhang W, Pang H, Gao X et al (2006) Pituitary adenylate cyclase-activating polypeptide (PACAP) 38 and PACAP4-6 are neuroprotective through inhibition of NADPH oxidase: potent regulators of microglia-mediated oxidative stress. J Pharmacol Exp Ther 319(2):595–603. doi:10.1124/jpet.106.102236
Acknowledgments
This work is supported by the National Natural Science Foundation of China (30770745, 81071015, 81571229), the National Key Research and Development Program of China (2016YFC1306000, 2016YFC1306300), the Natural Science Foundation of Bei**g, China (7082032), the National Key Basic Research Program of China (2011CB504100), the Key Project of Natural Science Foundation of Bei**g, China (B) (kz201610025030), the Key Project of Natural Science Foundation of Bei**g, China (4161004, kz200910025001), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (2013BAI09B03), the Project of Bei**g Institute for Brain Disorders (BIBD-PXM2013_014226_07_000084), High Level Technical Personnel Training Project of Bei**g Health System, China (2009-3-26), the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Bei**g Municipality (IDHT20140514), Capital Clinical Characteristic Application Research (Z1211070010 12161), the Bei**g Healthcare Research Project, China (JING-15-2, JING-15-3), Excellent Personnel Training Project of Bei**g, China (20071D0300400076), Important National Science & Technology Specific Projects ( 2011ZX09102-003-01), Key Project of National Natural Science Foundation of China (81030062) and Basic-Clinical Research Cooperation Funding of Capital Medical University (2015-JL-PT-X04, 10JL49, 14JL15), and Youth Research Fund, Bei**g Tiantan Hospital, Capital Medical University, China (2014-YQN-YS-18, 2015-YQN-15, 2015-YQN-05, 2015-YQN-14, 2015-YQN-17).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Our research involves animal participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Not applicable.
Additional information
This work was performed at Bei**g Tiantan Hospital, Capital Medical University, Bei**g, China
Electronic Supplementary Material
ESM 1
(DOC 60 kb)
Rights and permissions
About this article
Cite this article
Zhang, W., Gao, Jh., Yan, Zf. et al. Minimally Toxic Dose of Lipopolysaccharide and α-Synuclein Oligomer Elicit Synergistic Dopaminergic Neurodegeneration: Role and Mechanism of Microglial NOX2 Activation. Mol Neurobiol 55, 619–632 (2018). https://doi.org/10.1007/s12035-016-0308-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-016-0308-2