Abstract
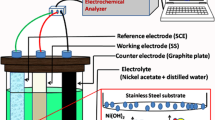
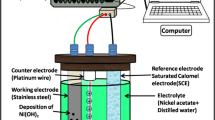
In the present investigation, we report the synthesis of ruthenium oxide (RuO2 · nH2O) thin films by simple chemical bath deposition (CBD) method at low temperature on the stainless steel substrate. The prepared thin films are characterized for their structural and morphological properties by means of X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT–IR) and scanning electron microscopy (SEM). The structural study revealed that the ruthenium oxide thin films are amorphous. Scanning electron microscopy study shows compact morphology with small overgrown particles on the surface of the substrate. FT–IR study confirms the formation of RuO2 · nH2O material. The supercapacitor behaviour of RuO2 · nH2O thin film was studied using cyclic voltammetry (CV) technique in 0 · 5 M H2SO4electrolyte. RuO2 · nH2O film showed maximum specific capacitance of 192 F · g− 1at a scan rate of 20 mV · s− 1. The charge–discharge studies of RuO2 · nH2O carried out at 300 μA · cm− 2current density revealed the specific power of 1 · 5 kW.kg− 1and specific energy of 41 · 6 Wh.kg− 1with 95% coulombic efficiency.









Similar content being viewed by others
References
Ahn Y R, Song M Y, Jo S M, Park C R and Kim D Y 2006 Nanotechnology 17 2865
Arabale G, Wagh D, Kulkarni M, Mulla I S, Vernekar S P, Vijayymohanan K and Rao A M 2003 Chem. Phys. Lett. 376 207
Broughton J N and Brett M J 2002 Electrochem. Solid–State Lett. 5 A279
Chen W C, Wen T C and Teng H 2003 Electrochem. Acta 48 641
Chen L, Yuan C, Gao B, Chen S and Zhang X 2009 J. Solid–State Electrochem. 13 1925
Conway B E and Pell Dec W G 1998 Proc of 8th International Seminar on Double Layer Capacitors and Similar Energy Storage Devices (Florida: Deerfiled Beach)
Dandekar M S, Arabale G and Vijayymohanan K 2005 J. Power Sources 141 (2005), 198
Dhawale D S, Dubal D P, Jamadade V S, Salunkhe R R and Lokhande C D 2010 Curr. Appl. Phys. 10 904
Ganesh V, Pitchumani S and Lakshminarayanan V 2006 J. Power Sources 158 1523
Gujar T P, KimWY, Puspitasari I, Jung K D and Joo O S 2007 Int. J. Electrochem. Sci. 2 666
Huang L M, Lin H Z, Wen T C and Gopalan A 2006 Electrochim. Acta 52 1058
Hu C C and Chen W C 2004 Electrochem. Acta 49 3469
Hu C C and Huang Y H 1999 J. Electrochem. Soc. 146 246
Jow J J, Lee H J, Chen H R, Wu M S and Wei T Y 2007 Electrochim. Acta 52 2625
Karuppuchamy S and Jeong J M 2006 J. Oleo. Sci. 55 263
Kim I H and Kim K B 2001 Electrochem. Solid–State Lett. 4 A62
Kim H and Kim K B 2006 J. Electrochem. Soc. 153 A383
Komura T, Sakabayashi H and Takahashi K 1995 Bull. Chem. Soc. Jpn. 68 476
Mane R S and Lokhande C D 2000 Mater. Chem. Phys. 65 1
Mink J, Kristof J, Battisti A D, Daalio S and Nemeth C 1995 Surf. Sci. 335 252
Mondal K S, Prasad R K and Munichandraiah N 2005 Synth. Met. 148 275
Park B O, Lokhande C D, Park H, Jung K and Joo O S 2004a J. Mater. Sci. 39 4313
Park B O, Lokhande C D, Park H S, Jung K D and Joo O S 2004b J. Power Sources 134 148
Patake V D and Lokhande C D 2007 Appl. Surf. Sci. 254 2820
Pawar S M, Pawar B S, Kim J H, Joo O S and Lokhande C D 2011 Curr. Appl. Phys. 11 117
Pico F, Ibanez J, Lillo-Rodenas M A, Linares-Solano A, Rojas R M, Amarilla J M and Rojo J M 2008 J. Power Sources 176 417
Prasad K R and Munichandraiah N 2002 Electrochem. Solid–State Lett. 5 271
Sugimoto W, Iwata H, Murakami Y and Takasu Y 2004 J. Electrochem. Soc. 151 A1181
Wang Y and Herron N 1991 J. Phys. Chem. 95 525
Wang C C and Hu C C 2004 Mater. Chem. Phys. 83 289
Yu N and Gao L 2009 Electrochem. Commun. 11 220
Zheng J P, Cygan P J and Jow T R 1995 J. Electrochem. Soc. 142 2699
Acknowledgments
One of the authors (PRD) is thankful to UGC, New Delhi, for financial support through a UGC-Research fellowship for meritorious student. Authors are also grateful to the Council for Scientific and Industrial Research (CSIR), New Delhi (India) for financial support through the scheme no. 03(1165)/10/EMR-II.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
DESHMUKH, P.R., PUSAWALE, S.N., BULAKHE, R.N. et al. Supercapacitive performance of hydrous ruthenium oxide (RuO2 · nH2O) thin films synthesized by chemical route at low temperature. Bull Mater Sci 36, 1171–1176 (2013). https://doi.org/10.1007/s12034-013-0592-7
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12034-013-0592-7