Abstract
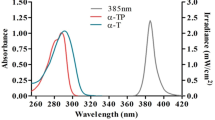
With the increasing levels of atmospheric ozone depletion, there has been much concern about the causal effects of high levels of ultraviolet radiation reaching the Earth’s surface on skin cancer. This has led to growing interest in identifying new active ingredients for use in commercial sunscreens. In our study, the chemical compound 2-benzoyl-3-phenylquinoxaline 1,4-dioxide (BPQ) prepared by the Beirut reaction was tested for its ability to protect a human keratinocyte cell line (HaCaT) against ultraviolet B radiation (280–315 nm). We show that BPQ exhibited strong absorbance in the UVB range, with an overall absorption spectrum very similar to that of Padimate-O, a well-known active ingredient used in commercial sunscreens. HaCaT cells, which were irradiated with UVB in the presence of multiple doses of BPQ, exhibited, in a dose-dependent fashion, a significantly higher viability and lower oxidative stress levels than cells irradiated in the absence of drug. Our results show that BPQ is a potential photoprotective drug that holds great promise for use as an active ingredient in commercial sunscreens.






Similar content being viewed by others
References
Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978.
Besaratinia A, Synold TW, Chen H, Chang C, Riggs AD, Pfeifer GP, Cleaver JE. DNA lesions induced by UV A1 and B radiation in human cells: comparative analyses in the overall genome and in the p53 tumor suppressor gene. Proc Natl Acad Sci USA. 2005;102:10058–63.
Pfeifer GP, You YH, Besaratinia A. Mutations induced by ultraviolet light. Mutat Res. 2005;571:19–31.
Ehrhart JC, Gosselet FP, Culerrier RM, Sarasin A. UVB-induced mutations in human key gatekeeper genes governing signalling pathways and consequences for skin tumorigenesis. Photochem Photobiol Sci. 2003;2:825–34.
Manney GL, Santee ML, Rex M, Livesey NJ, Pitts MC, Veekfind P, Nash ER, Wohltmann I, Lehmann R, Froidevaux L, Poole LR, Schoeberl MR, Haffner DP, Davies J, Dorokhov V, Gernandt H, Johnson B, Kivi R, Kyrö E, Larsen N, Levelt PF, Makshtas A, McElroy CT, Nakajima H, Parrondo MC, Tarasick DW, Gathen P, Walker KA, Zinoviev NS. Unprecedented Arctic ozone loss in 2011. Nature. 2011;478:469.
Garcia RR. Atmospheric science: an Arctic ozone hole? Nature. 2011;478:462.
Peak MJ, Peak JG, Moehring MP, Webb RB. Ultraviolet action spectra for DNA dimer induction, lethality, and mutagenesis in Escherichia coli with emphasis on the UVB region. Photochem Photobiol. 1984;40:613–20.
Kielbassa C, Roza L, Epe B. Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis. 1997;18:811–6.
Ziegler A, Remington L, Brash DE, Kimmelman J, Sharma HW, Jonason AS, Simon JA, Jacks T, Leffellt DJ. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6.
Vitale N, Kisslinger A, Paladino S, Procaccini C, Matarese G, Pierantoni GM, Mancini FP, Tramontano D. Resveratrol couples apoptosis with autophagy in UVB-irradiated HaCaT cells. PloS ONE. 2013. doi:10.1371/journal.pone.0080728.
Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2000;16:195–201.
Ryser S, Schuppli M, Gauthier B, Hernandez DR, Roye O, Hohl D, Moodycliffe AM. UVB-induced skin inflammation and cutaneous tissue injury is dependent on the MHC class I-like protein, CD1d. J Invest Dermatol. 2014;134:192–202.
Cheng G, Li B, Wang C, Zhang H, Liang G, Weng Z, Hao H, Wang X, Liu Z, Dai M, Wang Y, Yuan Z. Systematic and molecular basis of the antibacterial action of quinoxaline 1,4-di-N-oxides against Escherichia coli. PLoS ONE. 2015. doi:10.1371/journal.pone.0136450.
Hennessey TD, Edwards JR. Antibacterial properties of quindoxin: a new growth-promoting agent. Vet Rec. 1972;90:187–91.
Carta A, Corona P, Loriga M. Quinoxaline 1, 4-dioxide: a versatile scaffold endowed with manifold activities. Curr Med Chem. 2005;12:2259–72.
Diab-Assaf M, Haddadin MJ, Yared P, Assaad C, Gali-Muhtasib HU. Quinoxaline 1,4-dioxides: hypoxia selective therapeutic agents. Mol carcinogen. 2002;33:198–205.
Gali-Muhtasib H, Sidani M, Geara F, Mona AD, Al-Hmaira J, Haddadin MJ, Zaatari G. Quinoxaline 1,4-dioxides are novel angiogenesis inhibitors that potentiate antitumor effects of ionizing radiation. Int J Oncol. 2004;24:1121.
Ghattass K, El-Sitt S, Zibara K, Rayes S, Haddadin MJ, El-Sabban M, Gali-Muhtasib H. The quinoxaline di-N-oxide DCQ blocks breast cancer metastasis in vitro and in vivo by targeting the hypoxia inducible factor-1 pathway. Mol Cancer. 2014;13:1–12.
Haddadin MJ, Issidorides CH. Photolysis of a quinoxaline-di-N-oxide. Tetrahedron Lett. 1967;8:753–6.
Gali-Muhtasib HU, Haddadin MJ, Nazer MZ, Sodir NM, Maalouf SW. Photoprotective effects of some quinoxaline 1,4-dioxides in hairless mice. Med Oncol. 1998;15:262–9.
Haddadin MJ, Issidorides CH. The beirut reaction. Heterocycles. 1993;35:1503.
Vostálová J, Zdařilová A, Svobodová A. Prunella vulgaris extract and rosmarinic acid prevent UVB-induced DNA damage and oxidative stress in HaCaT keratinocytes. Arch Dermatol Res. 2010;302:171–81.
Velasco MV, Sarruf FD, Salgado-Santos IM, Haroutiounian-Filho CA, Kaneko TM, Baby AR. Broad-spectrum bioactive sunscreens. Int J Pharm. 2008;363:50–7.
Acknowledgments
This work was funded by undergraduate research experience (URE) grant award from the Faculty of Arts and Sciences, American University of Beirut. We are grateful to the members of the Kamal Shair Central Science Research Laboratory for their technical help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Mouawad, J., Saadeh, F., Tabosh, H.A. et al. The photoprotective effects of 2-benzoyl-3-phenylquinoxaline 1,4-dioxide against UVB-induced damage in HaCaT cells. Med Oncol 33, 86 (2016). https://doi.org/10.1007/s12032-016-0802-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-016-0802-4