Abstract
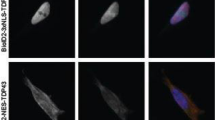
Prion protein (PrP) is a ubiquitous conserved glycoprotein predominantly expressed in neurons of the central nervous system (CNS). To elucidate on its cellular function, we performed a yeast two-hybrid screen within an adult human brain cDNA library for potential PrP-binding molecules. A novel protein, HS-1 associated protein X-1 (HAX-1), was identified to be able to bind with PrP strongly. The interaction between the two proteins has been further verified by glutathione-S-transferase (GST) pull-down and immunoprecipitation assays. The minimal binding regions were mapped to the segments of residues aa 91–163 for PrPC and residues aa 38–129 for HAX-1. Immunofluorescent assays of co-expressions of human PrP and HAX-1 in 293T and SHSY-5Y cells revealed marked co-localizations of those two proteins in cytoplasm. Moreover, the co-expression of HAX-1 and wild-type PrP (PG5) was found to enhance the cellular resistance to the challenge of H2O2. Contrarily, co-transfection of HAX-1 did not reverse but aggravated the cytotoxicities of the genetic CJD (gCJD) associated PrP mutants with nine- (PG9) and fourteen-octarepeats (PG14). Our data provide for the first time a new PrP-interacting partner that may play role in cell oxidative stress and anti-apoptosis physiologically and cell damage pathologically.






Similar content being viewed by others
References
An R, Dong C, Lei Y et al (2008) PrP mutants with different numbers of octarepeat sequences are more susceptible to the oxidative stress. Sci China C Life Sci 51:630–639
Brown DR, Schulz-Schaeffer WJ, Schmidt B, Kretzschmar HA (1997) Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp Neurol 146:104–112
Bueler H, Fischer M, Lang Y et al (1992) Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577–582
Bueler H, Aguzzi A, Sailer A et al (1993) Mice devoid of PrP are resistant to scrapie. Cell 73:1339–1347
Carlsson G, van’t Hooft I, Melin M et al (2008) Central nervous system involvement in severe congenital neutropenia: neurological and neuropsychological abnormalities associated with specific HAX1 mutations. J Intern Med 264:388–400
Chao JR, Parganas E, Boyd K, Hong CY, Opferman JT, Ihle JN (2008) Hax1-mediated processing of HtrA2 by Parl allows survival of lymphocytes and neurons. Nature 452:98–102
Chen L, Yang Y, Han J et al (2007) Removal of the glycosylation of prion protein provokes apoptosis in SF126. J Biochem Mol Biol 40:662–669
Chen JM, Gao C, Shi Q et al (2008) Different expression patterns of CK2 subunits in the brains of experimental animals and patients with transmissible spongiform encephalopathies. Arch Virol 153:1013–1020
Collinge J (1999) Variant Creutzfeldt–Jakob disease. Lancet 354:317–323
Collinge J, Whittington MA, Sidle KC et al (1994) Prion protein is necessary for normal synaptic function. Nature 370:295–297
Cuille J, Chelle PL (1939) Experimental transmission of trembling to the goat. In pp. 1058–1060
Dong CF, Wang XF, Wang X et al (2008a) Molecular interaction between prion protein and GFAP both in native and recombinant forms in vitro. Med Microbiol Immunol 197:361–368
Dong CF, Shi S, Wang XF et al (2008b) The N-terminus of PrP is responsible for interacting with tubulin and fCJD related PrP mutants possess stronger inhibitive effect on microtubule assembly in vitro. Arch Biochem Biophys 470:83–92
Dufva M, Olsson M, Rymo L (2001) Epstein-Barr virus nuclear antigen 5 interacts with HAX-1, a possible component of the B-cell receptor signalling pathway. J Gen Virol 82:1581–1587
Fadeel B, Grzybowska E (2009) HAX-1: a multifunctional protein with emerging roles in human disease. Biochim Biophys Acta 1790:1139–1148
Gajdusek DC, Gibbs CJ, Alpers M (1966) Experimental transmission of a Kuru-like syndrome to chimpanzees. Nature 209:794–796
Gallagher AR, Cedzich A, Gretz N, Somlo S, Witzgall R (2000) The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc Natl Acad Sci USA 97:4017–4022
Grassi J, Comoy E, Simon S et al (2001) Rapid test for the preclinical postmortem diagnosis of BSE in central nervous system tissue. Vet Rec 149:577–582
Han L, Wan YZ, Han J et al (2007) Preliminary analyses for influence of mutant PrPs with different number of octapeptide. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 21:208–210
Hayward PA, Bell JE, Ironside JW (1994) Prion protein immunocytochemistry: reliable protocols for the investigation of Creutzfeldt–Jakob disease. Neuropathol Appl Neurobiol 20:375–383
Hegde RS, Mastrianni JA, Scott MR et al (1998) A transmembrane form of the prion protein in neurodegenerative disease. Science 279:827–834
Hegde RS, Tremblay P, Groth D, DeArmond SJ, Prusiner SB, Lingappa VR (1999) Transmissible and genetic prion diseases share a common pathway of neurodegeneration. Nature 402:822–826
Hundt C, Peyrin JM, Haik S et al (2001) Identification of interaction domains of the prion protein with its 37-kDa/67-kDa laminin receptor. EMBO J 20:5876–5886
Kawaguchi Y, Nakajima K, Igarashi M et al (2000) Interaction of Epstein-Barr virus nuclear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J Virol 74:10104–10111
Keshet GI, Bar-Peled O, Yaffe D, Nudel U, Gabizon R (2000) The cellular prion protein colocalizes with the dystroglycan complex in the brain. J Neurochem 75:1889–1897
Kretzschmar HA, Prusiner SB, Stowring LE, DeArmond SJ (1986) Scrapie prion proteins are synthesized in neurons. Am J Pathol 122:1–5
Kurschner C, Morgan JI (1996) Analysis of interaction sites in homo- and heteromeric complexes containing Bcl-2 family members and the cellular prion protein. Brain Res Mol Brain Res 37:249–258
Kuwahara C, Takeuchi AM, Nishimura T et al (1999) Prions prevent neuronal cell-line death. Nature 400:225–226
Lee HG, Park SJ, Choi EK, Carp RI, Kim YS (1999) Increased expression of prion protein is associated with changes in dopamine metabolism and MAO activity in PC12 cells. J Mol Neurosci 13:121–126
Lees DM, Hart IR, Marshall JF (2008) Existence of multiple isoforms of HS1-associated protein X-1 in murine and human tissues. J Mol Biol 379:645–655
Mange A, Milhavet O, Umlauf D, Harris D, Lehmann S (2002) PrP-dependent cell adhesion in N2a neuroblastoma cells. FEBS Lett 514:159–162
Manson J, West JD, Thomson V, McBride P, Kaufman MH, Hope J (1992) The prion protein gene: a role in mouse embryogenesis? Development 115:117–122
Milhavet O, Lehmann S (2002) Oxidative stress and the prion protein in transmissible spongiform encephalopathies. Brain Res Brain Res Rev 38:328–339
Morrissey MP, Shakhnovich EI (1999) Evidence for the role of PrP(C) helix 1 in the hydrophilic seeding of prion aggregates. Proc Natl Acad Sci USA 96:11293–11298
Mouillet-Richard S, Ermonval M, Chebassier C et al (2000) Signal transduction through prion protein. Science 289:1925–1928
Prusiner SB (1998) Prions. Proc Natl Acad Sci USA 95:13363–13383
Rambold AS, Miesbauer M, Rapaport D et al (2006) Association of Bcl-2 with misfolded prion protein is linked to the toxic potential of cytosolic PrP. Mol Biol Cell 17:3356–3368
Rangel A, Madronal N, Gruart A et al (2009) Regulation of GABA(A) and glutamate receptor expression, synaptic facilitation and long-term potentiation in the hippocampus of prion mutant mice. PLoS ONE 4:e7592
Rieger R, Edenhofer F, Lasmezas CI, Weiss S (1997) The human 37-kDa laminin receptor precursor interacts with the prion protein in eukaryotic cells. Nat Med 3:1383–1388
Sailer A, Bueler H, Fischer M, Aguzzi A, Weissmann C (1994) No propagation of prions in mice devoid of PrP. Cell 77:967–968
Schmitt-Ulms G, Legname G, Baldwin MA et al (2001) Binding of neural cell adhesion molecules (N-CAMs) to the cellular prion protein. J Mol Biol 314:1209–1225
Sharp TV, Wang HW, Koumi A et al (2002) K15 protein of Kaposi’s sarcoma-associated herpesvirus is latently expressed and binds to HAX-1, a protein with antiapoptotic function. J Virol 76:802–816
Shmerling D, Hegyi I, Fischer M et al (1998) Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93:203–214
Speare JO, Rush TS 3rd, Bloom ME, Caughey B (2003) The role of helix 1 aspartates and salt bridges in the stability and conversion of prion protein. J Biol Chem 278:12522–12529
Spielhaupter C, Schatzl HM (2001) PrPC directly interacts with proteins involved in signaling pathways. J Biol Chem 276:44604–44612
Stahl N, Borchelt DR, Hsiao K, Prusiner SB (1987) Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51:229–240
Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deuschle U, Watanabe T (1997) HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol 158:2736–2744
Vafiadaki E, Sanoudou D, Arvanitis DA, Catino DH, Kranias EG, Kontrogianni-Konstantopoulos A (2007) Phospholamban interacts with HAX-1, a mitochondrial protein with anti-apoptotic function. J Mol Biol 367:65–79
Vafiadaki E, Arvanitis DA, Pagakis SN et al (2009) The anti-apoptotic protein HAX-1 interacts with SERCA2 and regulates its protein levels to promote cell survival. Mol Biol Cell 20:306–318
Wang XF, Dong CF, Zhang J et al (2008) Human tau protein forms complex with PrP and some GSS- and fCJD-related PrP mutants possess stronger binding activities with tau in vitro. Mol Cell Biochem 310:49–55
Watzlawik J, Skora L, Frense D et al (2006) Prion protein helix1 promotes aggregation but is not converted into beta-sheet. J Biol Chem 281:30242–30250
Weiss S, Proske D, Neumann M, Groschup MH, Kretzschmar HA et al (1997) RNA aptamers specifically interact with the prion protein PrP. J Virol 71:8790–8797
White AR, Collins SJ, Maher F et al (1999) Prion protein-deficient neurons reveal lower glutathione reductase activity and increased susceptibility to hydrogen peroxide toxicity. Am J Pathol 155:1723–1730
Yedavalli VS, Shih HM, Chiang YP et al (2005) Human immunodeficiency virus type 1 Vpr interacts with antiapoptotic mitochondrial protein HAX-1. J Virol 79:13735–13746
Zeng F, Watt NT, Walmsley AR, Hooper NM (2003) Tethering the N-terminus of the prion protein compromises the cellular response to oxidative stress. J Neurochem 84:480–490
Acknowledgements
This work was supported by Chinese National Natural Science Foundation Grants 30771914 and 30800975, Institution Technique R&D Grant (2008EG150300), National Basic Research Program of China (973 Program) (2007CB310505), China Mega-Project for Infectious Disease (2009ZX10004-101), and the SKLID Development Grant (2008SKLID102 and 2008SKLID202).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
The primers for amplifying various lengths of human PrP and HAX-1 (DOC 45 kb)
Rights and permissions
About this article
Cite this article
**g, YY., Li, XL., Shi, Q. et al. A Novel PrP Partner HS-1 Associated Protein X-1 (HAX-1) Protected the Cultured Cells Against the Challenge of H2O2 . J Mol Neurosci 45, 216–228 (2011). https://doi.org/10.1007/s12031-011-9498-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-011-9498-2