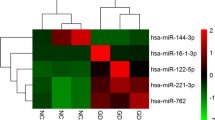
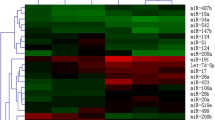
Abstract
Purpose
It has been increasingly suggested that specific microRNAs expression profiles in the circulation and atrial tissue are associated with the susceptibility to atrial fibrillation. Nonetheless, the role of circulating microRNAs in Graves’ disease patients with atrial fibrillation has not yet been well described. The objective of the study was to identify the role of circulating microRNAs as specific biomarkers for the diagnosis of Graves’ disease with atrial fibrillation.
Methods
The expression profiles of eight serum microRNAs, which are found to be critical in the pathogenesis of atrial fibrillation, were determined in patients with Graves’ disease with or without atrial fibrillation. MicroRNA expression analysis was performed by real-time PCR in normal control subjects (NC; n = 17), patients with Graves’ disease without atrial fibrillation (GD; n = 29), patients with Graves’ disease with atrial fibrillation (GD + AF; n = 14), and euthyroid patients with atrial fibrillation (AF; n = 22).
Results
Three of the eight serum microRNAs,i.e., miR-1a, miR-26a, and miR-133, had significantly different expression profiles among the four groups. Spearman’s correlation analysis showed that the relative expression level of miR-1a was positively correlated with free triiodothyronine (FT3) and free thyroxine (FT4), and negatively related to thyroid stimulating hormone. Spearman’s correlations analysis also revealed that the level of miR-1a was negatively correlated with a critical echocardiographic parameter (left atrial diameter), which was dramatically increased in GD + AF group compared to GD group. Furthermore, the receiver-operating characteristic curve analysis indicated that, among the eight microRNAs, miR-1a had the largest area under the receiver-operating characteristic curves not only for discriminating between individuals with and without Graves’ disease, but also for predicting the presence of atrial fibrillation in patients with Graves’ disease.
Conclusions
Our findings showed that the levels of serum miR-1a were significantly decreased in GD + AF group compared with GD group, suggesting that serum miR-1a might serve as a novel biomarker for diagnosis of atrial fibrillation in patients with Graves’ disease.




Similar content being viewed by others
References
M. den Hoed, M. Eijgelsheim, T. Esko, B.J. Brundel, D.S. Peal, D.M. Evans, I.M. Nolte, A.V. Segre, H. Holm, R.E. Handsaker, H.J. Westra, T. Johnson, A. Isaacs, J. Yang, A. Lundby, J.H. Zhao, Y.J. Kim, M.J. Go, P. Almgren, M. Bochud, G. Boucher, M.C. Cornelis, D. Gudbjartsson, D. Hadley, P. van der Harst, C. Hayward, M. den Heijer, W. Igl, A.U. Jackson, Z. Kutalik, J. Luan, J.P. Kemp, K. Kristiansson, C. Ladenvall, M. Lorentzon, M.E. Montasser, O.T. Njajou, P.F. O’Reilly, S. Padmanabhan, B. St Pourcain, T. Rankinen, P. Salo, T. Tanaka, N.J. Timpson, V. Vitart, L. Waite, W. Wheeler, W. Zhang, H.H. Draisma, M.F. Feitosa, K.F. Kerr, P.A. Lind, E. Mihailov, N.C. Onland-Moret, C. Song, M.N. Weedon, W. **e, L. Yengo, D. Absher, C.M. Albert, A. Alonso, D.E. Arking, P.I. de Bakker, B. Balkau, C. Barlassina, P. Benaglio, J.C. Bis, N. Bouatia-Naji, S. Brage, S.J. Chanock, P.S. Chines, M. Chung, D. Darbar, C. Dina, M. Dorr, P. Elliott, S.B. Felix, K. Fischer, C. Fuchsberger, E.J. de Geus, P. Goyette, V. Gudnason, T.B. Harris, A.L. Hartikainen, A.S. Havulinna, S.R. Heckbert, A.A. Hicks, A. Hofman, S. Holewijn, F. Hoogstra-Berends, J.J. Hottenga, M.K. Jensen, A. Johansson, J. Junttila, S. Kaab, B. Kanon, S. Ketkar, K.T. Khaw, J.W. Knowles, A.S. Kooner, J.A. Kors, M. Kumari, L. Milani, P. Laiho, E.G. Lakatta, C. Langenberg, M. Leusink, Y. Liu, R.N. Luben, K.L. Lunetta, S.N. Lynch, M.R. Markus, P. Marques-Vidal, I. Mateo Leach, W.L. McArdle, S.A. McCarroll, S.E. Medland, K.A. Miller, G.W. Montgomery, A.C. Morrison, M. Muller-Nurasyid, P. Navarro, M. Nelis, J.R. O’Connell, C.J. O’Donnell, K.K. Ong, A.B. Newman, A. Peters, O. Polasek, A. Pouta, P.P. Pramstaller, B.M. Psaty, D.C. Rao, S.M. Ring, E.J. Rossin, D. Rudan, S. Sanna, R.A. Scott, J.S. Sehmi, S. Sharp, J.T. Shin, A.B. Singleton, A.V. Smith, N. Soranzo, T.D. Spector, C. Stewart, H.M. Stringham, K.V. Tarasov, A.G. Uitterlinden, L. Vandenput, S.J. Hwang, J.B. Whitfield, C. Wijmenga, S.H. Wild, G. Willemsen, J.F. Wilson, J.C. Witteman, A. Wong, Q. Wong, Y. Jamshidi, P. Zitting, J.M. Boer, D.I. Boomsma, I.B. Borecki, C.M. van Duijn, U. Ekelund, N.G. Forouhi, P. Froguel, A. Hingorani, E. Ingelsson, M. Kivimaki, R.A. Kronmal, D. Kuh, L. Lind, N.G. Martin, B.A. Oostra, N.L. Pedersen, T. Quertermous, J.I. Rotter, Y.T. van der Schouw, W.M. Verschuren, M. Walker, D. Albanes, D.O. Arnar, T.L. Assimes, S. Bandinelli, M. Boehnke, R.A. de Boer, C. Bouchard, W.L. Caulfield, J.C. Chambers, G. Curhan, D. Cusi, J. Eriksson, L. Ferrucci, W.H. van Gilst, N. Glorioso, J. de Graaf, L. Groop, U. Gyllensten, W.C. Hsueh, F.B. Hu, H.V. Huikuri, D.J. Hunter, C. Iribarren, B. Isomaa, M.R. Jarvelin, A. Jula, M. Kahonen, L.A. Kiemeney, M.M. van der Klauw, J.S. Kooner, P. Kraft, L. Iacoviello, T. Lehtimaki, M.L. Lokki, B.D. Mitchell, G. Navis, M.S. Nieminen, C. Ohlsson, N.R. Poulter, L. Qi, O.T. Raitakari, E.B. Rimm, J.D. Rioux, F. Rizzi, I. Rudan, V. Salomaa, P.S. Sever, D.C. Shields, A.R. Shuldiner, J. Sinisalo, A.V. Stanton, R.P. Stolk, D.P. Strachan, J.C. Tardif, U. Thorsteinsdottir, J. Tuomilehto, D.J. van Veldhuisen, J. Virtamo, J. Viikari, P. Vollenweider, G. Waeber, E. Widen, Y.S. Cho, J.V. Olsen, P.M. Visscher, C. Willer, L. Franke, B.C. Global, C.A. Consortium, J. Erdmann, J.R. Thompson, P.G. Consortium, A. Pfeufer, Q.G. Consortium, N. Sotoodehnia, Q.-I. Consortium, C. Newton-Cheh, C.-A. Consortium, P.T. Ellinor, B.H. Stricker, A. Metspalu, M. Perola, J.S. Beckmann, G.D. Smith, K. Stefansson, N.J. Wareham, P.B. Munroe, O.C. Sibon, D.J. Milan, H. Snieder, N.J. Samani, R.J. Loos, Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat. Genet. 45(6), 621–631 (2013)
C.T. Sawin, A. Geller, P.A. Wolf, A.J. Belanger, E. Baker, P. Bacharach, P.W. Wilson, E.J. Benjamin, R.B. D’Agostino, Low serum thyrotropin concentrations as a risk factor for atrial fibrillation in older persons. N. Engl. J. Med. 331(19), 1249–1252 (1994)
J. Heeringa, E.H. Hoogendoorn, W.M. van der Deure, A. Hofman, R.P. Peeters, W.C. Hop, M. den Heijer, T.J. Visser, J.C. Witteman, High-normal thyroid function and risk of atrial fibrillation: the Rotterdam study. Arch. Intern. Med. 168(20), 2219–2224 (2008)
M.D. Gammage, J.V. Parle, R.L. Holder, L.M. Roberts, F.D. Hobbs, S. Wilson, M.C. Sheppard, J.A. Franklyn, Association between serum free thyroxine concentration and atrial fibrillation. Arch. Intern. Med. 167(9), 928–934 (2007)
X. Luo, B. Yang, S. Nattel, MicroRNAs and atrial fibrillation: mechanisms and translational potential. Nat. Rev. Cardiol. 12(2), 80–90 (2015)
G. Santulli, G. Iaccarino, N. De Luca, B. Trimarco, G. Condorelli, Atrial fibrillation and microRNAs. Front. Physiol. 5, 15 (2014)
R. Liu, X. Ma, L. Xu, D. Wang, X. Jiang, W. Zhu, B. Cui, G. Ning, D. Lin, S. Wang, Differential microRNA expression in peripheral blood mononuclear cells from Graves’ disease patients. J. Clin. Endocrinol. Metab. 97(6), E968–E972 (2012)
R.M. O’Connell, D.S. Rao, A.A. Chaudhuri, D. Baltimore, Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10(2), 111–122 (2010)
M. Hulsmans, P. Sinnaeve, B. Van der Schueren, C. Mathieu, S. Janssens, P. Holvoet, Decreased miR-181a expression in monocytes of obese patients is associated with the occurrence of metabolic syndrome and coronary artery disease. J. Clin. Endocrinol. Metab. 97(7), E1213–E1218 (2012)
D.P. Bartel, MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2), 281–297 (2004)
S. Fichtlscherer, S. De Rosa, H. Fox, T. Schwietz, A. Fischer, C. Liebetrau, M. Weber, C.W. Hamm, T. Roxe, M. Muller-Ardogan, A. Bonauer, A.M. Zeiher, S. Dimmeler, Circulating microRNAs in patients with coronary artery disease. Circ. Res. 107(5), 677–684 (2010)
M. Karakas, C. Schulte, S. Appelbaum, F. Ojeda, K.J. Lackner, T. Munzel, R.B. Schnabel, S. Blankenberg, T. Zeller Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur. Heart. J. 38(7), 516–523 (2017)
M.A. Cortez, C. Bueso-Ramos, J. Ferdin, G. Lopez-Berestein, A.K. Sood, G.A. Calin, MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat. Rev. Clin. Oncol. 8(8), 467–477 (2011)
P.S. Mitchell, R.K. Parkin, E.M. Kroh, B.R. Fritz, S.K. Wyman, E.L. Pogosova-Agadjanyan, A. Peterson, J. Noteboom, K.C. O’Briant, A. Allen, D.W. Lin, N. Urban, C.W. Drescher, B.S. Knudsen, D.L. Stirewalt, R. Gentleman, R.L. Vessella, P.S. Nelson, D.B. Martin, M. Tewari, Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U. S. A. 105(30), 10513–10518 (2008)
Y. Zhao, J.F. Ransom, A. Li, V. Vedantham, M. von Drehle, A.N. Muth, T. Tsuchihashi, M.T. McManus, R.J. Schwartz, D. Srivastava, Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 129(2), 303–317 (2007)
O. Adam, B. Lohfelm, T. Thum, S.K. Gupta, S.L. Puhl, H.J. Schafers, M. Bohm, U. Laufs, Role of miR-21 in the pathogenesis of atrial fibrosis. Basic. Res. Cardiol. 107(5), 278 (2012)
Z. Girmatsion, P. Biliczki, A. Bonauer, G. Wimmer-Greinecker, M. Scherer, A. Moritz, A. Bukowska, A. Goette, S. Nattel, S.H. Hohnloser, J.R. Ehrlich, Changes in microRNA-1 expression and IK1 up-regulation in human atrial fibrillation. Heart. Rhythm. 6(12), 1802–1809 (2009)
X. Luo, Z. Pan, H. Shan, J. **ao, X. Sun, N. Wang, H. Lin, L. **ao, A. Maguy, X.Y. Qi, Y. Li, X. Gao, D. Dong, Y. Zhang, Y. Bai, J. Ai, L. Sun, H. Lu, X.Y. Luo, Z. Wang, Y. Lu, B. Yang, S. Nattel, MicroRNA-26 governs profibrillatory inward-rectifier potassium current changes in atrial fibrillation. J. Clin. Invest. 123(5), 1939–1951 (2013)
K. Dawson, R. Wakili, B. Ordog, S. Clauss, Y. Chen, Y. Iwasaki, N. Voigt, X.Y. Qi, M.F. Sinner, D. Dobrev, S. Kaab, S. Nattel, MicroRNA29: a mechanistic contributor and potential biomarker in atrial fibrillation. Circulation. 127(14), 1466–1475 (2013). 1475e1461-1428
R.F. Duisters, A.J. Tijsen, B. Schroen, J.J. Leenders, V. Lentink, I. van der Made, V. Herias, R.E. van Leeuwen, M.W. Schellings, P. Barenbrug, J.G. Maessen, S. Heymans, Y.M. Pinto, E.E. Creemers, miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ. Res. 104(2), 170–178 (2009). 176p following 178
N. Cooley, M.J. Cowley, R.C. Lin, S. Marasco, C. Wong, D.M. Kaye, A.M. Dart, E.A. Woodcock, Influence of atrial fibrillation on microRNA expression profiles in left and right atria from patients with valvular heart disease. Physiol. Genomics. 44(3), 211–219 (2012)
Y. Lu, Y. Zhang, N. Wang, Z. Pan, X. Gao, F. Zhang, Y. Zhang, H. Shan, X. Luo, Y. Bai, L. Sun, W. Song, C. Xu, Z. Wang, B. Yang, MicroRNA-328 contributes to adverse electrical remodeling in atrial fibrillation. Circulation. 122(23), 2378–2387 (2010)
T.Y. Ling, X.L. Wang, Q. Chai, T.W. Lau, C.M. Koestler, S.J. Park, R.C. Daly, K.L. Greason, J. Jen, L.Q. Wu, W.F. Shen, W.K. Shen, Y.M. Cha, H.C. Lee, Regulation of the SK3 channel by microRNA-499--potential role in atrial fibrillation. Heart. Rhythm. 10(7), 1001–1009 (2013)
C. Bernecker, L. Lenz, M.S. Ostapczuk, S. Schinner, H. Willenberg, M. Ehlers, S. Vordenbaumen, J. Feldkamp, M. Schott, MicroRNAs miR-146a1, miR-155_2, and miR-200a1 are regulated in autoimmune thyroid diseases. Thyroid. 22(12), 1294–1295 (2012)
European Heart Rhythm, A., European Association for Cardio-Thoracic, S., A.J. Camm, P. Kirchhof, G.Y. Lip, U. Schotten, I. Savelieva, S. Ernst, I.C. Van Gelder, N. Al-Attar, G. Hindricks, B. Prendergast, H. Heidbuchel, O. Alfieri, A. Angelini, D. Atar, P. Colonna, R. De Caterina, J. De Sutter, A. Goette, B. Gorenek, M. Heldal, S.H. Hohloser, P. Kolh, J.Y. Le Heuzey, P. Ponikowski, F.H. Rutten, Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur. Heart. J. 31(19), 2369–2429 (2010)
N. Hakimzadeh, A.Y. Nossent, A.M. van der Laan, S.H. Schirmer, M.W. de Ronde, S.J. Pinto-Sietsma, N. van Royen, P.H. Quax, I.E. Hoefer, J.J. Piek, Circulating MicroRNAs Characterizing Patients with Insufficient Coronary Collateral Artery Function. PLoS ONE 10(9), e0137035 (2015)
N.S. Lai, D.G. Wu, X.G. Fang, Y.C. Lin, S.S. Chen, Z.B. Li, S.S. Xu, Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br. J. Cancer. 112(7), 1241–1246 (2015)
M. **ang, Y. Zeng, R. Yang, H. Xu, Z. Chen, J. Zhong, H. **e, Y. Xu, X. Zeng, U6 is not a suitable endogenous control for the quantification of circulating microRNAs. Biochem. Biophys. Res. Commun. 454(1), 210–214 (2014)
X. Zhang, S. Shao, H. Geng, Y. Yu, C. Wang, Z. Liu, C. Yu, X. Jiang, Y. Deng, L. Gao, J. Zhao, Expression profiles of six circulating microRNAs critical to atherosclerosis in patients with subclinical hypothyroidism: a clinical study. J. Clin. Endocrinol. Metab. 99(5), E766–E774 (2014)
H. Wang, W. Peng, X. Ouyang, W. Li, Y. Dai, Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl. Res. 160(3), 198–206 (2012)
Y. Shao, H. Ren, C. Lv, X. Ma, C. Wu, Q. Wang, Changes of serum Mir-217 and the correlation with the severity in type 2 diabetes patients with different stages of diabetic kidney disease. Endocrine 55(1), 130–138 (2017)
L. Jiang, J. Huang, Y. Chen, Y. Yang, R. Li, Y. Li, X. Chen, D. Yang, Identification of several circulating microRNAs from a genome-wide circulating microRNA expression profile as potential biomarkers for impaired glucose metabolism in polycystic ovarian syndrome. Endocrine 53(1), 280–290 (2016)
M. Pelloni, G. Coltrinari, D. Paoli, F. Pallotti, F. Lombardo, A. Lenzi, L. Gandini Differential expression of miRNAs in the seminal plasma and serum of testicular cancer patients. Endocrine (2016). [Epub ahead of print]. doi:10.1007/s12020-016-1150-z
L. Shen, F. Huang, L. Ye, W. Zhu, X. Zhang, S. Wang, W. Wang, G. Ning, Circulating microRNA predicts insensitivity to glucocorticoid therapy in Graves’ ophthalmopathy. Endocrine 49(2), 445–456 (2015)
D.D. McManus, K. Tanriverdi, H. Lin, N. Esa, M. Kinno, D. Mandapati, S. Tam, O.N. Okike, P.T. Ellinor, J.F. Keaney Jr., J.K. Donahue, E.J. Benjamin, J.E. Freedman, Plasma microRNAs are associated with atrial fibrillation and change after catheter ablation (the miRhythm study). Heart. Rhythm. 12(1), 3–10 (2015)
Z. Liu, C. Zhou, Y. Liu, S. Wang, P. Ye, X. Miao, J. **a, The expression levels of plasma micoRNAs in atrial fibrillation patients. PLoS ONE 7(9), e44906 (2012)
Y. Goren, E. Meiri, C. Hogan, H. Mitchell, D. Lebanony, N. Salman, J.E. Schliamser, O. Amir, Relation of reduced expression of MiR-150 in platelets to atrial fibrillation in patients with chronic systolic heart failure. Am. J. Cardiol. 113(6), 976–981 (2014)
T. Liu, S. Zhong, F. Rao, Y. Xue, Z. Qi, S. Wu, Catheter ablation restores decreased plasma miR-409-3p and miR-432 in atrial fibrillation patients. Europace. 18(1), 92–99 (2016)
S. Fazio, E.A. Palmieri, G. Lombardi, B. Biondi, Effects of thyroid hormone on the cardiovascular system. Recent. Prog. Horm. Res. 59, 31–50 (2004)
W.H. Dillmann, Biochemical basis of thyroid hormone action in the heart. Am. J. Med. 88(6), 626–630 (1990)
M. Lagos-Quintana, R. Rauhut, A. Yalcin, J. Meyer, W. Lendeckel, T. Tuschl, Identification of tissue-specific microRNAs from mouse. Curr. Biol. 12(9), 735–739 (2002)
P.K. Rao, Y. Toyama, H.R. Chiang, S. Gupta, M. Bauer, R. Medvid, F. Reinhardt, R. Liao, M. Krieger, R. Jaenisch, H.F. Lodish, R. Blelloch, Loss of cardiac microRNA-mediated regulation leads to dilated cardiomyopathy and heart failure. Circ. Res. 105(6), 585–594 (2009)
D. Terentyev, A.E. Belevych, R. Terentyeva, M.M. Martin, G.E. Malana, D.E. Kuhn, M. Abdellatif, D.S. Feldman, T.S. Elton, S. Gyorke, miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ. Res. 104(4), 514–521 (2009)
Y. Lu, S. Hou, D. Huang, X. Luo, J. Zhang, J. Chen, W. Xu, Expression profile analysis of circulating microRNAs and their effects on ion channels in Chinese atrial fibrillation patients. Int. J. Clin. Exp. Med. 8(1), 845–853 (2015)
B. Yang, H. Lin, J. **ao, Y. Lu, X. Luo, B. Li, Y. Zhang, C. Xu, Y. Bai, H. Wang, G. Chen, Z. Wang, The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat. Med. 13(4), 486–491 (2007)
X. Jia, S. Zheng, X. **e, Y. Zhang, W. Wang, Z. Wang, Y. Zhang, J. Wang, M. Gao, Y. Hou, MicroRNA-1 accelerates the shortening of atrial effective refractory period by regulating KCNE1 and KCNB2 expression: an atrial tachypacing rabbit model. PLoS ONE 8(12), e85639 (2013)
F. Stillitano, G. Lonardo, G. Giunti, M. Del Lungo, R. Coppini, V. Spinelli, L. Sartiani, C. Poggesi, A. Mugelli, E. Cerbai, Chronic atrial fibrillation alters the functional properties of If in the human atrium. J. Cardiovasc. Electrophysiol. 24(12), 1391–1400 (2013)
H.D. Yu, S. **a, C.Q. Zha, S.B. Deng, J.L. Du, Q. She, Spironolactone Regulates HCN Protein Expression Through Micro-RNA-1 in Rats With Myocardial Infarction. J. Cardiovasc. Pharmacol. 65(6), 587–592 (2015)
Y.D. Li, Y.F. Hong, Y. Yusufuaji, B.P. Tang, X.H. Zhou, G.J. Xu, J.X. Li, L. Sun, J.H. Zhang, Q. **n, J. **ong, Y.T. Ji, Y. Zhang, Altered expression of hyperpolarization-activated cyclic nucleotide-gated channels and microRNA-1 and -133 in patients with age-associated atrial fibrillation. Mol. Med. Rep. 12(3), 3243–3248 (2015)
Funding
This study was supported by the National Natural Science Foundation of China (81400834), Young Talents Training Project of Tongji University (2013KJ092), and a grant of People’s Hospital of Shanghai Putuo District (RYK15-08).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of ethic committees of our hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Wang, F., Zhang, Sj., Yao, X. et al. Circulating microRNA-1a is a biomarker of Graves’ disease patients with atrial fibrillation. Endocrine 57, 125–137 (2017). https://doi.org/10.1007/s12020-017-1331-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-017-1331-4