Abstract
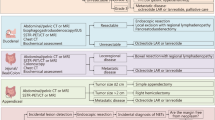
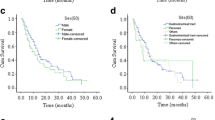
The incidence of neuroendocrine neoplasias (NENs), especially of the gastro-entero-pancreatic (GEP), system relatively increased over the past decades, as a result of advanced diagnostic tools, a better clinical awareness, and distinguished pathological diagnostic recognition. Previous reports hypothesized an increased risk for secondary malignancies in patients with NEN especially in GEP–NENs. The present study was designed to investigate the coincidence of NENs and secondary malignancies in a large patient collective. A retrospective analysis was performed on 161 patients (85 female and 76 male) with NEN of various origins. Clinical data of these patients, different classification systems (TNM/WHO), proliferations-based grading, and clinical follow-up were collected and analyzed. Out of 143 patients with a sporadic NEN, 15 (10.49 %) patients were identified with secondary malignant tumors. Median age at the time of the primary operation for NEN was 65 years, whereas the median age of initial diagnosis of associated tumors was 59 years. Mean follow-up time was 61 months. The risk of develo** a secondary malignancy was most elevated for patients with an NEN of the lung, the stomach, and the ileum (60, 50 and 20 %, respectively). The spectrum of secondary malignancies included various types of cancer. Kaplan–Meier survival analysis shows a difference suggesting that patients with a secondary malignancy demonstrate a worse survival compared to patients without a secondary tumor; no significance was detected (p = 0.349). Our data suggest that secondary malignancies in patients with NEN’s especially in GEP–NENs are found more frequently than in general population. Therefore, patients with NEN need a continuous and detailed follow-up. The reason for the increased incidence of secondary malignancies in patients with NENs remains to be elucidated.
Similar content being viewed by others
References
D. Dhall, R. Mertens, C. Bresee, R. Parakh, H.L. Wang, M. Li et al., Ki-67 proliferative index predicts progression-free survival of patients with well-differentiated ileal neuroendocrine tumors. Hum. Pathol. 43(4), 489–495 (2012)
J. Strosberg, A. Nasir, D. Coppola, M. Wick, L. Kvols, Correlation between grade and prognosis in metastatic gastroenteropancreatic neuroendocrine tumors. Hum. Pathol. 40(9), 1262–1268 (2009)
M. Krausch, F. Kroepil, N. Lehwald, A. Lachenmayer, M. Schott, M. Anlauf, K. Cupisti, W.T. Knoefel, A. Raffel, Notch 1 tumor expression is lacking in highly proliferative pancreatic neuroendocrine tumors. Endocrine (2012). doi:10.1007/s12020-012-9850-5
M. Anlauf, N. Garbrecht, J. Bauersfeld, A. Schmitt, T. Henopp, P. Komminoth, P.U. Heitz, A. Perren, G. Klöppel, Hereditary neuroendocrine tumors of the gastroenteropancreatic system. Virchows Arch. 451(Suppl 1), S29–S38 (2007)
C.S. Landry, C.R. Scoggins, K.M. McMasters, R.C. Martin 2nd, Management of hepatic metastasis of gastrointestinal carcinoid tumors. J. Surg. Oncol. 97(3), 253–258 (2008)
F. Costa, E. Domenichini, G. Garavito, R. Medrano, G. Mendez, J. O’Connor, W. Rojas, S. Torres, R.N. Younes, G. Delle Fave, K. Oberg, Management of neuroendocrine tumors: a meeting of experts from Latin America. Neuroendocrinology 88(3), 235–242 (2008)
K. Müssig, M.O. Oksüz, K. Dudziak, B. Ueberberg, M. Wehrmann, M. Horger, S. Schulz, H.U. Häring, C. Pfannenberg, R. Bares, B. Gallwitz, S. Petersenn, Association of somatostatin receptor 2 immunohistochemical expression with [111In]-DTPA octreotide scintigraphy and [68 Ga]-DOTATOC PET/CT in neuroendocrine tumors. Horm. Metab. Res. 42(8), 599–606 (2010)
K.L. Yim, Role of biological targeted therapies in gastroenteropancreatic neuroendocrine tumours. Endocrine 40(2), 181–186 (2011)
I.M. Modlin, S.F. Moss, B.I. Gustafsson, B. Lawrence, S. Schimmack, M. Kidd, The archaic distinction between functioning and nonfunctioning neuroendocrine neoplasms is no longer clinically relevant. Langenbecks Arch. Surg. 396(8), 1145–1156 (2011)
I.M. Modlin, S.F. Moss, D.C. Chung, R.T. Jensen, E. Snyderwine, Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J. Natl. Cancer Inst. 100(18), 1282–1289 (2008)
Krystallenia I. Alexandraki, Gregory Kaltsas, Gastroenteropancreatic neuroendocrine tumors: new insights in the diagnosis and therapy. Endocrine 41, 40–52 (2012)
F. Ehehalt, H.D. Saeger, C.M. Schmidt, R. Grutzmann, Neuroendocrine tumors of the pancreas. Oncologist. 14(5), 456–467 (2009)
A. Faggiano, P. Ferolla, F. Grimaldi, D. Campana, M. Manzoni, M.V. Davi et al., Natural history of gastro-entero-pancreatic and thoracic neuroendocrine tumors. Data from a large prospective and retrospective Italian Epidemiological study: THE NET MANAGEMENT STUDY. J. Endocrinol. Investig. 35(9), 817–823 (2012)
S. Massironi, V. Sciola, M. Peracchi, C. Ciafardini, M.P. Spampatti, D. Conte, Neuroendocrine tumors of the gastro-entero-pancreatic system. World J. Gastroenterol. 14(35), 5377–5384 (2008)
G. Rindi, C. Bordi, Endocrine tumours of the gastrointestinal tract: aetiology, molecular pathogenesis and genetics. Best Pract. Res. Clin. Gastroenterol. 19(4), 519–534 (2005)
R.G. Robertson, W.J. Geiger, N.B. Davis, Carcinoid tumors. Am. Fam. Physician 74(3), 429–434 (2006)
J.C. Yao, M. Hassan, A. Phan, C. Dagohoy, C. Leary, J.E. Mares et al., One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 26(18), 3063–3072 (2008)
G. Kloppel, G. Rindi, M. Anlauf, A. Perren, P. Komminoth, Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 451(Suppl 1), S9–S27 (2007)
G. Rindi, G. Kloppel, A. Couvelard, P. Komminoth, M. Korner, J.M. Lopes et al., TNM staging of midgut and hindgut (neuro) endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 451(4), 757–762 (2007)
G. Rindi, G. Kloppel, H. Alhman, M. Caplin, A. Couvelard, W.W. de Herder et al., TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 449(4), 395–401 (2006)
J.S. de Vries, S. de Vries, D.C. Aronson, D.K. Bosman, E.A. Rauws, A. Bosma et al., Choledochal cysts: age of presentation, symptoms, and late complications related to Todani’s classification. J. Pediatr. Surg. 37(11), 1568–1573 (2002)
M. Anlauf, P. Gerlach, M. Schott, A. Raffel, M. Krausch, W.T. Knoefel et al., Pathology of neuroendocrine neoplasms. Chirurg 82(7), 567–573 (2011)
F.C.F. Bosman, R. Hruban, N. Theise, WHO Classification of Tumours of the Digestive System (IARC Press, Lyon, France, 2010)
M. Brune, B. Gerdes, M. Koller, M. Rothmund, Neuroendocrine tumors of the gastrointestinal tract (NETGI) and second primary malignancies—which is dominant? Dtsch. Med. Wochenschr. 128(46), 2413–2417 (2003)
V. Fendrich, J. Waldmann, D.K. Bartsch, K. Schlosser, M. Rothmund, B. Gerdes, Multiple primary malignancies in patients with sporadic pancreatic endocrine tumors. J. Surg. Oncol. 97(7), 592–595 (2008)
J.D. Godwin 2nd, Carcinoid tumors. An analysis of 2,837 cases. Cancer 36(2), 560–569 (1975)
I.M. Modlin, A. Sandor, An analysis of 8305 cases of carcinoid tumors. Cancer 79(4), 813–829 (1997)
M.B. Niederle, B. Niederle, Diagnosis and treatment of gastroenteropancreatic neuroendocrine tumors: current data on a prospectively collected, retrospectively analyzed clinical multicenter investigation. Oncologist 16(5), 602–613 (2011)
U.F. Pape, U. Berndt, J. Muller-Nordhorn, M. Bohmig, S. Roll, M. Koch et al., Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr. Relat. Cancer 15(4), 1083–1097 (2008)
E.A. Perez, L.G. Koniaris, S.E. Snell, J.C. Gutierrez, W.E. Sumner 3rd, D.J. Lee et al., 7201 carcinoids: increasing incidence overall and disproportionate mortality in the elderly. World J. Surg. 31(5), 1022–1030 (2007)
R. Prommegger, C. Ensinger, P. Steiner, T. Sauper, C. Profanter, R. Margreiter, Neuroendocrine tumors and second primary malignancy—a relationship with clinical impact? Anticancer Res. 24(2C), 1049–1051 (2004)
KO. Shebani, WW. Souba, DM. Finkelstein, PC. Stark, KM. Elgadi, KK. Tanabe, et al., Prognosis and survival in patients with gastrointestinal tract carcinoid tumors. Ann. Surg. 229(6), 815–21, discussion 22–3 (1999)
Y. Kobayashi, H. Arimoto, S. Watanabe, Occurrence of multiple primary cancer at the National Cancer Center Hospital, 1962–1989. Jpn. J. Clin. Oncol. 21(3), 233–251 (1991)
J.T. Flannery, J.D. Boice Jr, S.S. Devesa, R.A. Kleinerman, R.E. Curtis, J.F. Fraumeni Jr, Cancer registration in Connecticut and the study of multiple primary cancers, 1935–1982. Natl. Cancer Inst. Monogr. 68, 13–24 (1985)
B.H. Lang, K.P. Wong, Risk factors for nonsynchronous second primary malignancy and related death in patients with differentiated thyroid carcinoma. Ann. Surg. Oncol. 18(13), 3559–3565 (2011)
M.L. Cote, A.S. Wenzlaff, P.A. Philip, A.G. Schwartz, Secondary cancers after a lung carcinoid primary: a population-based analysis. Lung Cancer 52(3), 273–279 (2006)
K.A. Zucker, W.E. Longo, I.M. Modlin, A.J. Bilchik, T.E. Adrian, Malignant diathesis from jejunal-ileal carcinoids. Am. J. Gastroenterol. 84(2), 182–186 (1989)
G.M. Dores, C. Metayer, R.E. Curtis, C.F. Lynch, E.A. Clarke, B. Glimelius et al., Second malignant neoplasms among long-term survivors of Hodgkin’s disease: a population-based evaluation over 25 years. J. Clin. Oncol. 20(16), 3484–3494 (2002)
L.B. Travis, S.D. Fossa, S.J. Schonfeld, M.L. McMaster, C.F. Lynch, H. Storm et al., Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J. Natl. Cancer Inst. 97(18), 1354–1365 (2005)
H.M. Schuller, H.P. Witschi, E. Nylen, P.A. Joshi, E. Correa, K.L. Becker, Pathobiology of lung tumors induced in hamsters by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the modulating effect of hyperoxia. Cancer Res. 50(6), 1960–1965 (1990)
P.J. Taddei, R.M. Howell, S. Krishnan, S.B. Scarboro, D. Mirkovic, W.D. Newhauser, Risk of second malignant neoplasm following proton versus intensity-modulated photon radiotherapies for hepatocellular carcinoma. Phys. Med. Biol. 55(23), 7055–7065 (2010)
J.D. Wright, C.M. St Clair, I. Deutsch, Wm Burke, P. Gorrochurn, X. Sun et al., Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer 116(10), 2486–2492 (2010)
V. D’Onghia, R. Leoncini, R. Carli, A. Santoro, S. Giglioni, F. Sorbellini et al., Circulating gastrin and ghrelin levels in patients with colorectal cancer: correlation with tumour stage, Helicobacter pylori infection and BMI. Biomed.Pharmacother. 61(2–3), 137–141 (2007)
A.S. Takhar, O. Eremin, S.A. Watson, The role of gastrin in colorectal carcinogenesis. Surg. J. Royal Coll. Surg. Edinb. Irel. 2(5), 251–257 (2004)
R.J. Bold, J. Ishizuka, C.M. Townsend Jr, J.C. Thompson, Gastrin stimulates growth of human colon cancer cells via a receptor other than CCK-A or CCK-B. Biochem. Biophys. Res. Commun. 202(3), 1222–1226 (1994)
C.M. Thorburn, G.D. Friedman, C.J. Dickinson, J.H. Vogelman, N. Orentreich, J. Parsonnet, Gastrin and colorectal cancer: a prospective study. Gastroenterology 115(2), 275–280 (1998)
T.J. Koh, D. Chen, Gastrin as a growth factor in the gastrointestinal tract. Regul. Pept. 93(1–3), 37–44 (2000)
R.J. Bold, J. Ishizuka, C.Z. Yao, C.M. Townsend Jr, J.C. Thompson, Bombesin stimulates in vitro growth of human breast cancer independent of estrogen receptors status. Anticancer Res. 18(6A), 4051–4056 (1998)
F. Cuttitta, D.N. Carney, J. Mulshine, T.W. Moody, J. Fedorko, A. Fischler et al., Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature 316(6031), 823–826 (1985)
P. Kuiper, H.W. Verspaget, I. Biemond, E.S. de Jonge-Muller, S. van Eeden, M.L. van Velthuysen et al., Expression and ligand binding of bombesin receptors in pulmonary and intestinal carcinoids. J. Endocrinol. Investig. 34(9), 665–670 (2011)
I.I. Schnirer, J.C. Yao, J.A. Ajani, Carcinoid—a comprehensive review. Acta Oncol. 42(7), 672–692 (2003)
A. Chaudhry, K. Oberg, A. Gobl, C.H. Heldin, K. Funa, Expression of transforming growth factors beta 1, beta 2, beta 3 in neuroendocrine tumors of the digestive system. Anticancer Res. 14(5B), 2085–2091 (1994)
G. Vitale, P.M. van Koetsveld, W.W. de Herder, K. van der Wansem, J.A. Janssen, A. Colao, G. Lombardi, S.W. Lamberts, L.J. Hofland, Effects of type I interferons on IGF-mediated autocrine/paracrine growth of human neuroendocrine tumor cells. Am. J. Physiol. Endocrinol. Metab. 296(3), 559–566 (2009)
R. Hrascan, N. Pecina-Slaus, T.N. Martic, J.F. Colic, K. Gall-Troselj, K. Pavelic et al., Analysis of selected genes in neuroendocrine tumours: insulinomas and phaeochromocytomas. J. Neuroendocrinol. 20(8), 1015–1022 (2008)
Acknowledgments
The authors are grateful to Dipl.-Math. Dr. Dieter Hafner, Institute for Pharmacology and Clinical Pharmacology, Chairman: Univ.-Prof. Dr. med. J. W. Fischer, Heinrich-Heine University Duesseldorf, for his help with the statistical analysis.
Conflict of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krausch, M., Raffel, A., Anlauf, M. et al. Secondary malignancy in patients with sporadic neuroendocrine neoplasia. Endocrine 44, 510–516 (2013). https://doi.org/10.1007/s12020-013-9911-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-013-9911-4