Abstract
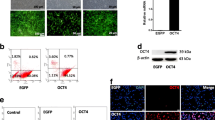
DNA methyltransferase (DNMT) inhibitor 5-aza-2’-deoxycytidine (5-aza-CdR) is able to cause DNA demethylation in the genome and induce the expression of silenced genes. Whether DNA demethylation can affect the gene expression of stem/progenitor cells has not been understood. Mouse utricle epithelia-derived progenitor cells (MUCs), which possess stem cell features as previously described, exhibit a potential DNA methylation status in the genome. In this study, MUCs were treated with 5-aza-CdR to determine whether DNMT inhibitor is able to induce the differentiation of MUCs. With 5-aza-CdR treatment for 72 hr, MUCs expressed epithelial genes including Cdh1, Krt8, Krt18, and Dsp. Further, hair cell genes Myo7a and Myo6 increased their expressions in response to 5-aza-CdR treatment. The decrease in the global methylated DNA values after 5-aza-CdR treatment indicated a significant DNA demethylation in the genome of MUCs, which may contribute to remarkably increased expression of epithelial genes and hair cell genes. The progenitor MUCs then turned into an epithelial-like hair cell fate with the expression of both epithelial and hair cell genes. This study suggests that stem cell differentiation can be stimulated by DNA demethylation, which may open avenues for studying stem cell fate induction using epigenetic approaches.




Similar content being viewed by others
References
Shi, F., & Edge, A. S. (2013). Prospects for replacement of auditory neurons by stem cells. Hearing Research, 297, 106–112.
Hu, Z., & Ulfendahl, M. (2013). The potential of stem cells for the restoration of auditory function in humans. Regenerative Medicine, 8(3), 309–318.
Groves, A. K., Zhang, K. D., & Fekete, D. M. (2013). The genetics of hair cell development and regeneration. Annual Review of Neuroscience, 36, 361–381.
Ronaghi, M., Nasr, M., & Heller, S. (2012). Concise review: Inner ear stem cells–an oxymoron, but why? Stem Cells, 30(1), 69–74.
Cotanche, D. A., & Kaiser, C. L. (2010). Hair cell fate decisions in cochlear development and regeneration. Hearing Research, 266(1–2), 18–25.
Okano, T., & Kelley, M. W. (2012). Stem cell therapy for the inner ear: recent advances and future directions. Trends in Amplification, 16(1), 4–18.
Li, H., Liu, H., & Heller, S. (2003). Pluripotent stem cells from the adult mouse inner ear. Nature Medicine, 9(10), 1293–1299.
Oshima, K., Grimm, C. M., Corrales, C. E., et al. (2007). Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. Journal of the Association for Research in Otolaryngology, 8(1), 18–31.
White, P. M., Doetzlhofer, A., Lee, Y. S., Groves, A. K., & Segil, N. (2006). Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature, 441(7096), 984–987.
Mizutari, K., Fujioka, M., Hosoya, M., et al. (2013). Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron, 77(1), 58–69.
Kelley, M. W., Talreja, D. R., & Corwin, J. T. (1995). Replacement of hair cells after laser microbeam irradiation in cultured organs of corti from embryonic and neonatal mice. The Journal of Neuroscience, 15(4), 3013–3026.
Zhang, L., & Hu, Z. (2012). Sensory epithelial cells acquire features of prosensory cells via epithelial to mesenchymal transition. Stem Cells and Development, 21(10), 1812–1821.
Oesterle, E. C., Campbell, S., Taylor, R. R., Forge, A., & Hume, C. R. (2008). Sox2 and JAGGED1 expression in normal and drug-damaged adult mouse inner ear. Journal of the Association for Research in Otolaryngology, 9(1), 65–89.
Batts, S. A., Shoemaker, C. R., & Raphael, Y. (2009). Notch signaling and Hes labeling in the normal and drug-damaged organ of Corti. Hearing Research, 249(1–2), 15–22.
Kelley, M. W. (2006). Regulation of cell fate in the sensory epithelia of the inner ear. Nature Reviews Neuroscience, 7(11), 837–849.
Zhang, L., & Hu, Z. (2012). Sensory Epithelial Cells Acquire Features of Prosensory Cells Via Epithelial to Mesenchymal Transition. Stem Cells and Development, 21(10), 1812–1821.
Barald, K. F., & Kelley, M. W. (2004). From placode to polarization: new tunes in inner ear development. Development, 131(17), 4119–4130.
Bird, A. (2007). Perceptions of epigenetics. Nature, 447(7143), 396–398.
Jones, P. A., & Takai, D. (2001). The role of DNA methylation in mammalian epigenetics. Science, 293(5532), 1068–1070.
Razin, A., & Cedar, H. (1991). DNA methylation and gene expression. Microbiological Reviews, 55(3), 451–458.
Bird, A. (2002). DNA methylation patterns and epigenetic memory. Genes and Development, 16(1), 6–21.
Dodge, J. E., Ramsahoye, B. H., Wo, Z. G., Okano, M., & Li, E. (2002). De novo methylation of MMLV provirus in embryonic stem cells: CpG versus non-CpG methylation. Gene, 289(1–2), 41–48.
Ghoshal, K., Datta, J., Majumder, S., et al. (2005). 5-Aza-deoxycytidine induces selective degradation of DNA methyltransferase 1 by a proteasomal pathway that requires the KEN box, bromo-adjacent homology domain, and nuclear localization signal. Molecular and Cellular Biology, 25(11), 4727–4741.
Klose, R. J., & Bird, A. P. (2006). Genomic DNA methylation: the mark and its mediators. Trends in Biochemical Sciences, 31(2), 89–97.
Auclair, G., & Weber, M. (2012). Mechanisms of DNA methylation and demethylation in mammals. Biochimie, 94(11), 2202–2211.
Phillips, T. (2008). The role of methylation in gene expression. Nature Education, 1(1), 116.
Sigalotti, L., Fratta, E., Coral, S., et al. (2007). Epigenetic drugs as pleiotropic agents in cancer treatment: biomolecular aspects and clinical applications. Journal of Cellular Physiology, 212(2), 330–344.
Sigalotti, L., Fratta, E., Coral, S., & Maio, M. (2014). Epigenetic drugs as immunomodulators for combination therapies in solid tumors. Pharmacology and Therapeutics, 142(3), 339–350.
Christman, J. K. (2002). 5-Azacytidine and 5-aza-2’-deoxycytidine as inhibitors of DNA methylation: mechanistic studies and their implications for cancer therapy. Oncogene, 21(35), 5483–5495.
Mossman, D., Kim, K. T., & Scott, R. J. (2010). Demethylation by 5-aza-2’-deoxycytidine in colorectal cancer cells targets genomic DNA whilst promoter CpG island methylation persists. BMC Cancer, 10, 366.
Patra, A., Deb, M., Dahiya, R., & Patra, S. K. (2011). 5-Aza-2’-deoxycytidine stress response and apoptosis in prostate cancer. Clinical Epigenetics, 2(2), 339–348.
Corn, P. G., Smith, B. D., Ruckdeschel, E. S., Douglas, D., Baylin, S. B., & Herman, J. G. (2000). E-cadherin expression is silenced by 5’ CpG island methylation in acute leukemia. Clinical Cancer Research, 6(11), 4243–4248.
Ling, Z. Q., Li, P., Ge, M. H., et al. (2011). Hypermethylation-modulated down-regulation of CDH1 expression contributes to the progression of esophageal cancer. International Journal of Molecular Medicine, 27(5), 625–635.
Lin, S. L. (2011). Concise review: Deciphering the mechanism behind induced pluripotent stem cell generation. Stem Cells, 29(11), 1645–1649.
Yan, X., Ehnert, S., Culmes, M., et al. (2014). 5-azacytidine improves the osteogenic differentiation potential of aged human adipose-derived mesenchymal stem cells by DNA demethylation. PloS One, 9(3), e90846.
Liang, G., Gonzales, F. A., Jones, P. A., Orntoft, T. F., & Thykjaer, T. (2002). Analysis of gene induction in human fibroblasts and bladder cancer cells exposed to the methylation inhibitor 5-aza-2’-deoxycytidine. Cancer Research, 62(4), 961–966.
Bennett, L. B., Schnabel, J. L., Kelchen, J. M., et al. (2009). DNA hypermethylation accompanied by transcriptional repression in follicular lymphoma. Genes, Chromosomes & Cancer, 48(9), 828–841.
Almstedt, M., Blagitko-Dorfs, N., Duque-Afonso, J., et al. (2010). The DNA demethylating agent 5-aza-2’-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leukemia Research, 34(7), 899–905.
Bender, C. M., Pao, M. M., & Jones, P. A. (1998). Inhibition of DNA methylation by 5-aza-2’-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Research, 58(1), 95–101.
Mund, C., Hackanson, B., Stresemann, C., Lubbert, M., & Lyko, F. (2005). Characterization of DNA demethylation effects induced by 5-Aza-2’-deoxycytidine in patients with myelodysplastic syndrome. Cancer Research, 65(16), 7086–7090.
De Smet, C., Lurquin, C., Lethe, B., Martelange, V., & Boon, T. (1999). DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Molecular and Cellular Biology, 19(11), 7327–7335.
Yamashita, S., Tsu**o, Y., Moriguchi, K., Tatematsu, M., & Ushijima, T. (2006). Chemical genomic screening for methylation-silenced genes in gastric cancer cell lines using 5-aza-2’-deoxycytidine treatment and oligonucleotide microarray. Cancer Science, 97(1), 64–71.
Acknowledgments
The authors thank Neelkumar Patel for his technical support and Jue Wang, Fei Nei, and **aoyang Li for valuable comments to the manuscript. The study is supported by NIDCD/NIH (1 R01 DC013275) and the Grants Plus Program from the Wayne State University.
Conflict of Interest
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Y., Hu, Z. Genome-Wide Demethylation by 5-aza-2’-Deoxycytidine Alters the Cell Fate of Stem/Progenitor Cells. Stem Cell Rev and Rep 11, 87–95 (2015). https://doi.org/10.1007/s12015-014-9542-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9542-z