Abstract
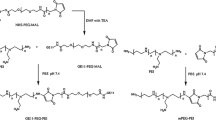
We developed a new magnetic nanovector to improve the efficiency and targeting of transgene therapy for oral squamous cell carcinoma (OSCC). Positively charged polymer PEI-modified Fe3O4 magnetic nanoparticles were tested as gene transfer vectors in the presence of a magnetic field. The Fe3O4 nanoparticles were prepared by a co-precipitation method and had good dispersibility in water. These nanoparticles modified by PEI were combined with negatively charged pACTERT-EGFP via electrostatic interaction. The transfection efficiency of the magnetic nano-gene vector with the magnetic field was determined by a fluorescence-inverted microscope and flow cytometry. The results showed significant improvement compared with the control group (p < 0.05). The magnetic complexes also exhibited up to 6-times higher transfection efficiency compared with commonly used PEI or lipofectin. On the basis of these results, the antitumor effect with suicide gene therapy using pACTERT-TRAIL in vitro and vivo was evaluated. In vitro apoptosis was determined with the Annexin V-FITC Apoptosis Detection Kit. The results suggested that PEI-modified Fe3O4 nanoparticles could mediate the killing of Tca83 cells. Furthermore, treatment with pACTERT-TRAIL delivered by magnetic nanoparticles showed a significant cytostatic effect through the induction of apoptosis in a xenograft model. This indicates that magnetic nano-gene vectors could improve the transgene efficiency for Tca83 cells and could exhibit antitumor functions with the plasmid pACTERT-TRAIL. This may be a new way to treat OSCC.







Similar content being viewed by others
Change history
30 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12013-022-01084-2
References
Shah, J. P., & Singh, B. (2006). Keynote comment: Why the lack of progress for oral cancer? The Lancet Oncology, 7, 356–357.
Gibson, M. K., & Forastiere, A. A. (2006). Reassessment of the role of induction chemotherapy for head and neck cancer. The Lancet Oncology, 7, 565–574.
Wiley, S. R., Schooley, K., Smolak, P. J., et al. (1995). Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity, 3, 673–682.
Pitti, R. M., Marsters, S. A., Ruppert, S., et al. (1996). Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. Journal of Biological Chemistry, 271, 12687–12690.
Kim, K., Fisher, M. J., Xu, S. Q., & El-Deiry, W. S. (2000). Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clinical Cancer Research, 6, 335–346.
Armeanu, S., Lauer, U. M., Smirnow, I., Schenk, M., Weiss, T. S., Gregor, M., et al. (2003). Adenoviral gene transfer of tumor necrosis factor–related apoptosis inducing ligand overcomes an impaired response of hepatoma cells but causes severe apoptosis in primary human hepatocytes. Cancer Research, 63, 2369–2372.
Jacob, D., Davis, J., Zhu, H., Zhang, L., Teraishi, F., & Wu, S. (2004). Suppressing orthotopic pancreatic tumor growth with a fiber-modified adenovector expressing the TRAIL gene from the human telomerase reverse transcriptase promoter. Clinical Cancer Research, 10, 3535–3541.
Griffith, T. S., Fialkov, J. M., Scott, D. L., Azuhata, T., Williams, R. D., Wall, N. R., et al. (2002). Induction and regulation of tumor necrosis factor–related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Research, 62, 3093–3099.
Voelkel-Johnson, C., King, D. L., & Norris, J. S. (2002). Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Therapy, 9, 164–172.
Yamanaka, T., Shiraki, K., Sugimoto, K., et al. (2000). Chemotherapeutic agents augment TRAIL-induced apoptosis in human hepatocellular carcinoma cell lines. Hepatology, 32, 482–490.
Kim, C. Y., Jeong, M., Mushiake, H., Kim, B. M., Kim, W. B., Ko, J. P., et al. (2006). Cancer gene therapy using a novel secretable trimeric TRAIL. Gene Therapy, 13, 330–338.
Argiris, K., Panethymitaki, C., & Tavassoli, M. (2011). Naturally occurring, tumor-specific, therapeutic proteins. Experimental Biology and Medicine (Maywood), 236, 524–536.
Gu, J., Kagawa, S., Takakura, M., Kyo, S., Inouse, M., Roth, J. A., et al. (2000). Tumorspecific transgene expression from the human telomerase reverse transcriptase promoter enables targeting of the therapeutic effects of the Bax gene to cancers. Cancer Research, 60, 5359–5364.
Hodes, R. (2001). Molecular targeting of cancer: Telomeres as targets. Proceedings of the National Academy of Sciences of the United States of America, 98, 7649–7651.
Liao, J., Mitsuyasu, T., Yamane, K., & Ohishi, M. (2000). Telomerase activity in oral and maxillofacial tumors. Oral Oncology, 36, 347–352.
Zang, G., Miao, L., Mu, Y., et al. (2009). Adenoviral mediated transduction of adenoid cystic carcinoma by human TRAIL gene driven with hTERT tumor-specific promoter induces apoptosis. Cancer Biology and Therapy, 8(10), 966–972.
Sanvicens, N., & Marco, M. P. (2008). Multifunctional nanoparticles–properties and prospects for their use in human medicine. Trends in Biotechnology, 26(8), 425–433.
Nehilla, Barrett J., Allen, Philip G., & Desai, Tejal A. (2008). Surfactant-free, drug-quantum-dot coloaded poly(lactide-co-glycolide) nanoparticles: Towards multifunctional nanoparticles. ACS Nano, 2(3), 538–544.
Wang, Lingyan, Luo, **, Schadt, Mark J., & Zhong, Chuan-Jian. (2010). Thin film assemblies of molecularly-linked metal nanoparticles and multifunctional properties. Langmuir, 26(2), 618–632.
Pellegrino, T., Kudera, S., Liedl, T., Muñoz Javier, A., Manna, L., & Parak, W. J. (2005). On the development of colloidal nanoparticles towards multifunctional structures and their possible use for biological applications. Small, 1(1), 48–63.
Lee, Mei-Hwa, Thomas, James L., Ho, Min-Hsien, Yuan, Ching, & Lin, Hung-Yin. (2010). Synthesis of magnetic molecularly imprinted poly(ethylene-co-vinyl alcohol) nanoparticles and their uses in the extraction and sensing of target molecules in urine. ACS Applied Materials and Interfaces, 2(6), 1729–1736.
Mahmoudi, Morteza, Simchi, Abdolreza, Imani, Mohammad, & Hfeli, Urs O. (2009). Superparamagnetic iron oxide nanoparticles with rigid cross-linked polyethylene glycol fumarate coating for application in imaging and drug delivery. Journal of Physical Chemistry C, 113(19), 8124–8131.
Williams, P. S., Carpino, F., & Zborowski, M. (2009). Magnetic nanoparticle drug carriers and their study by quadrupole magnetic field-flow fractionation. Molecular pharmaceutics, 6(5), 1290–1306.
Liong, M., Lu, J., Kovochich, M., **a, T., Ruehm, S. G., Nel, A. E., et al. (2008). Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano, 2(5), 889–896.
Gao, **hao, Hongwei, Gu, & Bing, Xu. (2009). Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Accounts of Chemical Research, 42(8), 1097–1107.
Mori, Kohsuke, Kondo, Yuichi, Morimoto, Shotaro, & Yamashita, Hiromi. (2008). Synthesis and multifunctional properties of superparamagnetic iron oxide nanoparticles coated with mesoporous silica involving single-site Ti oxide moiety. Journal of Physical Chemistry C, 112, 397–404.
Sun, C., Du, K., Fang, C., Bhattarai, N., Veiseh, O., Kievit, F., et al. (2010). PEG-mediated synthesis of highly dispersive multifunctional superparamagnetic nanoparticles: Their physicochemical properties and function in vivo. ACS Nano, 4(4), 2402–2410.
Jain, T., Morales, M., Sahoo, S., et al. (2005). Iron oxide nanoparticles for sustained delivery of anticancer agents. Molecular Pharmaceutics, 2(3), 194–205.
Son, S., Reichel, J., He, B., et al. (2005). Magnetic nanotubes for magnetic-field-assisted bioseparation, biointeraction, and drug delivery. Journal of the American Chemical Society, 127(20), 7316–7317.
Namgung, R., Singha, K., Yu, M. K., Jon, S., Kim, Y. S., Ahn, Y., et al. (2010). Hybrid superparamagnetic iron oxide nanoparticle-branched polyethylenimine magnetoplexes for gene transfection of vascular endothelial cells. Biomaterials, 31(14), 4204–4213.
Zheng, X., Lu, J., Deng, L., **ong, Y., & Chen, J. (2009). Preparation and characterization of magnetic cationic liposome in gene delivery. International Journal of Pharmaceutics, 366(1–2), 211–217.
Kievit, F. M., Veiseh, O., Fang, C., Bhattarai, N., Lee, D., Ellenbogen, R. G., et al. (2010). Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano, 4(8), 4587–4594.
Scherer, F., Anton, M., Schillinger, U., et al. (2002). Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Therapy, 9(2), 102–109.
Moore, A., Marecos, E., Bogdanov, A, Jr, et al. (2000). Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology, 214(2), 568–574.
Kamau, W., Hassa, P., Steitz, B., et al. (2006). Enhancement of the efficiency of non-viral gene delivery by application of pulsed magnetic field. Nucleic Acids Research, 34(5), e40.
Kadota, S., Kanayama, T., Miyajima, N., et al. (2005). Enhancing of measles virus infection by magnetofection. Journal of Virological Methods, 128(1–2), 61–66.
Morishita, N., Nakagami, H., Morishita, R., et al. (2005). Magnetic nanoparticles with surface modification enhanced gene delivery of HVJ-E vector. Biochemical and Biophysical Research Communications, 334(4), 1121–1126.
Plank, C., Anton, M., Rudolph, C., et al. (2003). Enhancing and targeting nucleic acid delivery by magnetic force. Expert Opinion on Biological Therapy, 3, 745–758.
Boussif, O., Lezoualc’h, F., Zanta, M. A., Mergny, M. D., Scherman, D., Demeneix, B., et al. (1995). A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proceedings of the National Academy of Sciences of the United States of America, 92(16), 7297–7301.
Miao, L., Zhang, K., Qiao, C., **, X., Zheng, C., Yang, B., et al. (2013). Antitumor effect of human TRAIL on adenoid cystic carcinoma using magnetic nanoparticle-mediated gene expression. Nanomedicine: Nanotechnology, Biology, and Medicine, 9(1), 141–150.
Lee, H., Lee, E., Kim do, K., Jang, N. K., Jeong, Y. Y., & Jon, S. (2006). Antibiofouling polymer-coated superparamagnetic iron oxide nanoparticles as potential magnetic resonance contrast agents for in vivo cancer imaging. Journal of the American Chemical Society, 128, 7383–7389.
Pan, X., Guan, J., Yoo, J. W., Epstein, A. J., Lee, L. J., & Lee, R. J. (2008). Cationic lipid coated magnetic nanoparticles associated with transferring for gene delivery. International Journal of Pharmaceutics, 358, 263–270.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No: 81300852, 30672338, 30740420551 and 30830108), Jiangsu Province Natural Science Foundation of China (BK20130079), the Youth Start Fund of Nan**g City (No. 2011-19-198*), the Third Level Fund for the Young Talents in the Health Field of Nan**g City. We thank Dr. Shenglin Li at Peking University, China for a gift of the Tca83 cell line.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Leiying Miao and Chao Liu have contributed to this work equally.
Rights and permissions
About this article
Cite this article
Miao, L., Liu, C., Ge, J. et al. Antitumor Effect of TRAIL on Oral Squamous Cell Carcinoma using Magnetic Nanoparticle-Mediated Gene Expression. Cell Biochem Biophys 69, 663–672 (2014). https://doi.org/10.1007/s12013-014-9849-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-9849-z