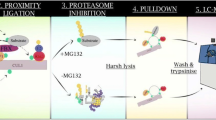
Abstract
The ubiquitin–proteasome system (UPS) plays a central role in maintaining protein homeostasis, emphasized by a myriad of diseases that are associated with altered UPS function such as cancer, muscle-wasting, and neurodegeneration. Protein ubiquitination plays a central role in both the promotion of proteasomal degradation as well as cellular signaling through regulation of the stability of transcription factors and other signaling molecules. Substrate-specificity is a critical regulatory step of ubiquitination and is mediated by ubiquitin ligases. Recent studies implicate ubiquitin ligases in multiple models of cardiac diseases such as cardiac hypertrophy, atrophy, and ischemia/reperfusion injury, both in a cardioprotective and maladaptive role. Therefore, identifying physiological substrates of cardiac ubiquitin ligases provides both mechanistic insights into heart disease as well as possible therapeutic targets. Current methods identifying substrates for ubiquitin ligases rely heavily upon non-physiologic in vitro methods, impeding the unbiased discovery of physiological substrates in relevant model systems. Here we describe a novel method for identifying ubiquitin ligase substrates utilizing tandem ubiquitin binding entities technology, two-dimensional differential in gel electrophoresis, and mass spectrometry, validated by the identification of both known and novel physiological substrates of the ubiquitin ligase MuRF1 in primary cardiomyocytes. This method can be applied to any ubiquitin ligase, both in normal and disease model systems, in order to identify relevant physiological substrates under various biological conditions, opening the door to a clearer mechanistic understanding of ubiquitin ligase function and broadening their potential as therapeutic targets.




Similar content being viewed by others
References
Colland, F. (2010). The therapeutic potential of deubiquitinating enzyme inhibitors. Biochemical Society Transactions, 38(Pt 1), 137–143.
Lim, K. H., & Baek, K. H. (2013). Deubiquitinating enzymes as therapeutic targets in cancer. Current Pharmaceutical Design, 19(22), 4039–4052.
Ikeda, F., & Dikic, I. (2008). Atypical ubiquitin chains: new molecular signals. “Protein modifications: beyond the usual suspects” review series. EMBO Reports, 9(6), 536–542.
Edelmann, M. J., Nicholson, B., & Kessler, B. M. (2011). Pharmacological targets in the ubiquitin system offer new ways of treating cancer, neurodegenerative disorders and infectious diseases. Expert Reviews in Molecular Medicine, 13, e35.
Zolk, O., Schenke, C., & Sarikas, A. (2006). The ubiquitin-proteasome system: focus on the heart. Cardiovascular Research, 70(3), 410–421.
Wilkie, Neil and Davies S. Drug Discovery World, Drug Discovery and Development News.
Centner, T., Yano, J., Kimura, E., McElhinny, A. S., Pelin, K., Witt, C. C., et al. (2001). Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. Journal of Molecular Biology, 306(4), 717–726.
Spencer, J. A., Eliazer, S., Ilaria, R. L., Richardson, J. A., & Olson, E. N. (2000). Regulation of microtubule dynamics and myogenic differentiation by MURF, a striated muscle RING-finger protein. The Journal of cell biology, 150(4), 771–784.
Kedar, V., McDonough, H., Arya, R., Li, H–. H., Rockman, H. A., & Patterson, C. (2004). Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proceedings of the National Academy of Sciences of the United States of America, 101(52), 18135–18140.
Polge, C., Heng, A.-E., Jarzaguet, M., Ventadour, S., Claustre, A., Combaret, L., et al. (2011). Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB Journal, 25(11), 3790–3802.
Willis, M. S., Zungu, M., & Patterson, C. (2010). Cardiac muscle ring finger-1–friend or foe? Trends in Cardiovascular Medicine, 20(1), 12–16.
Mearini, G., Schlossarek, S., Willis, M. S., & Carrier, L. (2008). The ubiquitin-proteasome system in cardiac dysfunction. Biochimica et Biophysica Acta, 1782(12), 749–763.
Powell, S. R., Herrmann, J., Lerman, A., Patterson, C., & Wang, X. (2012). The ubiquitin-proteasome system and cardiovascular disease. Progress in Molecular Biology and Translational Science, 109, 295–346.
Powell, S. R. (2006). The ubiquitin-proteasome system in cardiac physiology and pathology. American Journal of Physiology - Heart and Circulatory Physiology, 291(1), H1–H19.
Zhang, Y., Zeng, Y., Wang, M., Tian, C., Ma, X., Chen, H., et al. (2011). Cardiac-specific overexpression of E3 ligase Nrdp1 increases ischemia and reperfusion-induced cardiac injury. Basic Research in Cardiology, 106(3), 371–383.
Li, H–. H., Du, J., Fan, Y.-N., Zhang, M.-L., Liu, D.-P., Li, L., et al. (2011). The ubiquitin ligase MuRF1 protects against cardiac ischemia/reperfusion injury by its proteasome-dependent degradation of phospho-c-Jun. The American journal of pathology, 178(3), 1043–1058.
Yu, X., & Kem, D. C. (2010). Proteasome inhibition during myocardial infarction. Cardiovascular Research, 85(2), 312–320.
Nowis, D., Maczewski, M., Mackiewicz, U., Kujawa, M., Ratajska, A., Wieckowski, M. R., et al. (2010). Cardiotoxicity of the anticancer therapeutic agent bortezomib. The American Journal of Pathology, 176(6), 2658–2668.
Deshaies, R. J., & Joazeiro, C. A. P. (2009). RING domain E3 ubiquitin ligases. Annual Review of Biochemistry, 78, 399–434.
Wu, Z., Chen, Y., Yang, T., Gao, Q., Yuan, M., & Ma, L. (2012). Targeted ubiquitination and degradation of G-protein-coupled receptor kinase 5 by the DDB1-CUL4 ubiquitin ligase complex. PLoS One, 7(8), e43997.
Peschiaroli, A., Dorrello, N. V., Guardavaccaro, D., Venere, M., Halazonetis, T., Sherman, N. E., et al. (2006). SCFbetaTrCP-mediated degradation of Claspin regulates recovery from the DNA replication checkpoint response. Molecular Cell, 23(3), 319–329.
Kus, B., Gajadhar, A., Stanger, K., Cho, R., Sun, W., Rouleau, N., et al. (2005). A high throughput screen to identify substrates for the ubiquitin ligase Rsp5. The Journal of Biological Chemistry, 280(33), 29470–29478.
Loch, C. M., Eddins, M. J., & Strickler, J. E. (2011). Protein microarrays for the identification of praja1 e3 ubiquitin ligase substrates. Cell Biochemistry and Biophysics, 60(1–2), 127–135.
Qian, S.-B., McDonough, H., Boellmann, F., Cyr, D. M., & Patterson, C. (2006). CHIP-mediated stress recovery by sequential ubiquitination of substrates and Hsp70. Nature, 440(7083), 551–555.
Hjerpe, R., Aillet, F., Lopitz-Otsoa, F., Lang, V., England, P., & Rodriguez, M. S. (2009). Efficient protection and isolation of ubiquitylated proteins using tandem ubiquitin-binding entities. EMBO Reports, 10(11), 1250–1258.
Toraason, M., Luken, M. E., Breitenstein, M., Krueger, J. A., & Biagini, R. E. (1989). Comparative toxicity of allylamine and acrolein in cultured myocytes and fibroblasts from neonatal rat heart. Toxicology, 56(1), 107–117.
Osorio, C., Sullivan, P. M., He, D. N., Mace, B. E., Ervin, J. F., Strittmatter, W. J., et al. (2007). Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiology of Aging, 28(12), 1853–1862.
Jiang, J., Ballinger, C. A., Wu, Y., Dai, Q., Cyr, D. M., Höhfeld, J., et al. (2001). CHIP is a U-box-dependent E3 ubiquitin ligase: identification of Hsc70 as a target for ubiquitylation. The Journal of Biological Chemistry, 276(46), 42938–42944.
Willis, M. S., Schisler, J. C., Li, L., Rodríguez, J. E., Hilliard, E. G., Charles, P. C., et al. (2009). Cardiac muscle ring finger-1 increases susceptibility to heart failure in vivo. Circulation Research, 105(1), 80–88.
Witt, S. H., Granzier, H., Witt, C. C., & Labeit, S. (2005). MURF-1 and MURF-2 target a specific subset of myofibrillar proteins redundantly: towards understanding MURF-dependent muscle ubiquitination. Journal of Molecular Biology, 350(4), 713–722.
Kim, W., Bennett, E. J., Huttlin, E. L., Guo, A., Li, J., Possemato, A., et al. (2011). Systematic and quantitative assessment of the ubiquitin-modified proteome. Molecular Cell, 44(2), 325–340.
Cohen, S., Brault, J. J., Gygi, S. P., Glass, D. J., Valenzuela, D. M., Gartner, C., et al. (2009). During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. The Journal of Cell Biology, 185(6), 1083–1095.
Wagner, S. A., Beli, P., Weinert, B. T., Nielsen, M. L., Cox, J., Mann, M., et al. (2011). A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Molecular and Cellular Proteomics, 10(10), M111.013284.
Portbury, A. L., Willis, M. S., & Patterson, C. (2011). Tearin’ up my heart: proteolysis in the cardiac sarcomere. The Journal of Biological Chemistry, 286(12), 9929–9934.
David, Y., Ternette, N., Edelmann, M. J., Ziv, T., Gayer, B., Sertchook, R., et al. (2011). E3 ligases determine ubiquitination site and conjugate type by enforcing specificity on E2 enzymes. The Journal of Biological Chemistry, 286(51), 44104–44115.
Hoeller, D., Hecker, C.-M., Wagner, S., Rogov, V., Dötsch, V., & Dikic, I. (2007). E3-independent monoubiquitination of ubiquitin-binding proteins. Molecular Cell, 26(6), 891–898.
Friedman, D. B., Hoving, S., & Westermeier, R. (2009). Isoelectric focusing and two-dimensional gel electrophoresis. Methods in Enzymology, 463, 515–540.
Zhu, W., Smith, J. W., & Huang, C.-M. (2010). Mass spectrometry-based label-free quantitative proteomics. Journal of Biomedicine and Biotechnology, 2010, 840518.
Acknowledgments
The authors would like to thank the Michael Hooker Proteomics Center at UNC for protein identification services and Andrea Portbury for her critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jonathan C. Schisler and Carrie E. Rubel contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rubel, C.E., Schisler, J.C., Hamlett, E.D. et al. Diggin′ on U(biquitin): A Novel Method for the Identification of Physiological E3 Ubiquitin Ligase Substrates. Cell Biochem Biophys 67, 127–138 (2013). https://doi.org/10.1007/s12013-013-9624-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9624-6