Abstract
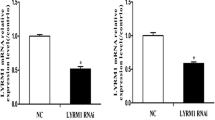
Fatty acid–binding protein 3 (FABP3) facilitates the movement of fatty acids in cardiac muscle. Previously, we reported that FABP3 is highly upregulated in the myocardium of ventricular septal defect patients and overexpression of FABP3 inhibited proliferation and promoted apoptosis in embryonic carcinoma cells (P19 cells). In this study, we aimed to investigate the effect of FABP3 gene silencing on P19 cell differentiation, proliferation and apoptosis. We used RNA interference and a lentiviral-based vector system to create a stable FABP3-silenced P19 cell line; knockdown of FABP3 was confirmed by quantitative real-time PCR. Expression analysis of specific differentiation marker genes using quantitative real-time PCR and observation of morphological changes using an inverted microscope revealed that knockdown of FABP3 did not significantly affect the differentiation of P19 cells into cardiomyocytes. CCK-8 proliferation assays and cell cycle analysis demonstrated that FABP3 gene silencing significantly inhibited P19 cell proliferation. Furthermore, Annexin V-FITC/propidium iodide staining and the caspase-3 activity assay revealed that FABP3 gene silencing significantly promoted serum starvation–induced apoptosis in P19 cells. In agreement with our previous research, these results demonstrate that FABP3 may play an important role during embryonic heart development, and that either overexpression or silencing of FABP3 will lead to an imbalance between proliferation and apoptosis, which may result in embryonic cardiac malformations.





Similar content being viewed by others
References
Olson, E. N. (2006). Gene regulatory networks in the evolution and development of the heart. Science, 313, 1922–1927.
Hoffman, J. I., & Kaplan, S. (2002). The incidence of congenital heart disease. Journal of the American College of Cardiology, 39, 1890–1900.
Garg, V. (2006). Insights into the genetic basis of congenital heart disease. Cellular and Molecular Life Sciences, 63, 1141–1148.
Sands, A. J., Casey, F. A., Craig, B. G., Dornan, J. C., Rogers, J., & Mulholland, H. C. (1999). Incidence and risk factors for ventricular septal defect in “low risk” neonates. Archives of Disease in Childhood. Fetal and Neonatal Edition, 81, F61–F63.
Kaynak, B., von Heydebreck, A., Mebus, S., Seelow, D., Hennig, S., Vogel, J., et al. (2003). Genome-wide array analysis of normal and malformed human hearts. Circulation, 107, 2467–2474.
Besnard, P., Niot, I., Poirier, H., Clement, L., & Bernard, A. (2002). New insights into the fatty acid-binding protein (FABP) family in the small intestine. Molecular and Cellular Biochemistry, 239, 139–147.
Qian, Q., Kuo, L., Yu, Y. T., & Rottman, J. N. (1999). A concise promoter region of the heart fatty acid-binding protein gene dictates tissue-appropriate expression. Circulation Research, 84, 276–289.
Zhang, H., Zhou, L., Yang, R., Sheng, Y., Sun, W., Kong, X., et al. (2006). Identification of differentially expressed genes in human heart with ventricular septal defect using suppression subtractive hybridization. Biochemical and Biophysical Research Communications, 342, 135–144.
Zhu, C., Hu, D. L., Liu, Y. Q., Zhang, Q. J., Chen, F. K., Kong, X. Q., et al. (2011). Fabp3 inhibits proliferation and promotes apoptosis of embryonic myocardial cells. Cell Biochemistry and Biophysics, 60, 259–266.
van der Heyden, M. A., van Kempen, M. J., Tsuji, Y., Rook, M. B., Jongsma, H. J., & Opthof, T. (2003). P19 embryonal carcinoma cells: A suitable model system for cardiac electrophysiological differentiation at the molecular and functional level. Cardiovascular Research, 58, 410–422.
Grepin, C., Nemer, G., & Nemer, M. (1997). Enhanced cardiogenesis in embryonic stem cells overexpressing the GATA-4 transcription factor. Development, 124, 2387–2395.
Chen, C., & Okayama, H. (1987). High-efficiency transformation of mammalian cells by plasmid DNA. Molecular and Cellular Biology, 7, 2745–2752.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C (T)) Method. Methods, 25, 402–408.
Vermes, I., Haanen, C., Steffens-Nakken, H., & Reutelingsperger, C. (1995). A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. Journal of Immunological Methods, 184, 39–51.
Koike, M., Sakaki, S., Amano, Y., & Kurosawa, H. (2007). Characterization of embryoid bodies of mouse embryonic stem cells formed under various culture conditions and estimation of differentiation status of such bodies. Journal of Bioscience and Bioengineering, 104, 294–299.
Lints, T. J., Parsons, L. M., Hartley, L., Lyons, I., & Harvey, R. P. (1993). Nkx-2.5: A novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development, 119, 419–431.
Bruneau, B. G., Nemer, G., Schmitt, J. P., Charron, F., Robitaille, L., Caron, S., et al. (2001). A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell, 106, 709–721.
Watt, A. J., Battle, M. A., Li, J., & Duncan, S. A. (2004). GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America, 101, 12573–12578.
Tang, M. K., Kindler, P. M., Cai, D. Q., Chow, P. H., Li, M., & Lee, K. K. (2004). Heart-type fatty acid binding proteins are upregulated during terminal differentiation of mouse cardiomyocytes, as revealed by proteomic analysis. Cell and Tissue Research, 316, 339–347.
Aagaard, L., & Rossi, J. J. (2007). RNAi therapeutics: Principles, prospects and challenges. Advanced Drug Delivery Reviews, 59, 75–86.
Dykxhoorn, D. M., Novina, C. D., & Sharp, P. A. (2003). Killing the messenger: Short RNAs that silence gene expression. Nature Reviews Molecular Cell Biology, 4, 457–467.
Abbas-Terki, T., Blanco-Bose, W., Deglon, N., Pralong, W., & Aebischer, P. (2002). Lentiviral-mediated RNA interference. Human Gene Therapy, 13, 2197–2201.
Brummelkamp, T. R., Bernards, R., & Agami, R. (2002). A system for stable expression of short interfering RNAs in mammalian cells. Science, 296, 550–553.
Zhu, C., Hu, D. L., Liu, Y. Q., Zhang, Q. J., Chen, F. K., Kong, X. Q., et al. (2011). Fabp3 inhibits proliferation and promotes apoptosis of embryonic myocardial cells. Cell Biochemistry and Biophysics, 60, 259–266.
Shen, Y. H., Song, G. X., Liu, Y. Q., Sun, W., Zhou, L. J., Liu, H. L., et al. (2012). Silencing of FABP3 promotes apoptosis and induces mitochondrion impairment in embryonic carcinoma cells. Journal of Bioenergetics and Biomembranes, 44, 317–323.
Song, G. X., Shen, Y. H., Liu, Y. Q., Sun, W., Miao, L. P., Zhou, L. J., Liu, H. L., Yang, R., Kong, X. Q., Cao, K. J., Qian, L. M., & Sheng, Y. H. (2012). Overexpression of FABP3 promotes apoptosis through inducing mitochondrial impairment in embryonic cancer cells. Journal of Cellular Biochemistry, Jul 2. doi: 10.1002/jcb.24243. [Epub ahead of print].
Srivastava, D. (2006). Genetic regulation of cardiogenesis and congenital heart disease. Annual Review of Pathology: Mechanisms of Disease, 1, 199–213.
Fiorina, P., Corradi, D., Pinelli, S., Maestri, R., Lagrasta, C., Buscaglia, M., et al. (2004). Apoptotic/mytogenic pathways during human heart development. International Journal of Cardiology, 96, 409–417.
Levy, M., Maurey, C., Celermajer, D. S., Vouhe, P. R., Danel, C., Bonnet, D., et al. (2007). Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. Journal of the American College of Cardiology, 49, 803–810.
Gittenberger-de, G. A., Bartelings, M. M., Deruiter, M. C., & Poelmann, R. E. (2005). Basics of cardiac development for the understanding of congenital heart malformations. Pediatric Research, 57, 169–176.
Hoye, A. T., Davoren, J. E., Wipf, P., Fink, M. P., & Kagan, V. E. (2008). Targeting mitochondria. Accounts of Chemical Research, 41, 87–97.
McBurney, M. W., Jones-Villeneuve, E. M., Edwards, M. K., & Anderson, P. J. (1982). Control of muscle and neuronal differentiation in a cultured embryonal carcinoma cell line. Nature, 299, 165–167.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 81070138), the Natural Science Foundation of Jiangsu Province, China (No. BK2010582) and the Talent Foundation of Jiangsu Province, China (WSN-020).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yahui Shen, Guixian Song contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shen, Y., Song, G., Liu, Y. et al. Silencing of FABP3 Inhibits Proliferation and Promotes Apoptosis in Embryonic Carcinoma Cells. Cell Biochem Biophys 66, 139–146 (2013). https://doi.org/10.1007/s12013-012-9462-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9462-y