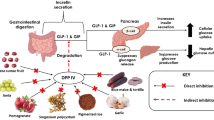
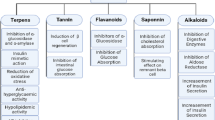
Abstract
Insulin resistance (IR) is a condition of impaired response of cells towards insulin. It is marked by excessive blood glucose, dysregulated insulin signalling, altered pathways, damaged pancreatic β-cells, metabolic disorders, etc. Chronic hyperglycemic conditions leads to type 2 diabetes mellitus (T2DM) which causes excess generation of highly reactive free radicals, causing oxidative stress, further leading to development and progression of complications like vascular dysfunction, damaged cellular proteins, and DNA. One of the causes for IR is dysregulation of protein tyrosine phosphatase 1B (PTP1B). Advancements in drug therapeutics have helped people manage IR by regulating PTP1B, however have been reported to cause side effects. Therefore, there is a growing interest on usage of phytochemical constituents having IR therapeutic properties and aiding to minimize these complications. Medicinal plants have not been utilized to their full potential as a therapeutic drug due to lack of knowledge of their active and effective chemical constituents, mode of action, regulation of IR parameters, and dosage of administration. This review highlights phytochemical constituents present in medicinal plants or spices, their potential effectiveness on proteins (PTP1B) regulating IR, and reported possible mechanism of action studied on in vitro models. The study gives current knowledge and future recommendations on the above aspects and is expected to be beneficial in develo** herbal drug using these phytochemical constituents, either alone or in combination, for medication of IR and diabetes.
Graphical Abstract


Similar content being viewed by others
Data Availability
NA.
References
Marques, A. M., Linhares, B. S., Dias Novaes, R., Freitas, M. B., Sarandy, M. M., & Gonçalves, R. V. (2020). Effects of the amount and type of carbohydrates used in type 2 diabetes diets in animal models: A systematic review. PLoS One1, 15(6), e0233364. https://doi.org/10.1371/journal.pone.0233364
Lenzen, S., Drinkgern, J., & Tiedge, M. (1996). Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radical Biology and Medicine, 20(3), 463–466. https://doi.org/10.1016/0891-5849(96)02051-5
Nathan, D. M. (1993). Long-term complications of diabetes mellitus. New England Journal of Medicine, 328(23), 1676–1685. https://doi.org/10.1056/NEJM199306103282306
Petersen, M. C., & Shulman, G. I. (2018, October 1). Mechanisms of insulin action and insulin resistance. Physiological Reviews. American Physiological Society. https://doi.org/10.1152/physrev.00063.2017
Panzhinskiy, E., Ren, J., & Nair, S. (2013). Protein tyrosine phosphatase 1B and insulin resistance: Role of endoplasmic reticulum stress/reactive oxygen species/nuclear factor kappa B axis. PLoS One1, 8(10), e77228. https://doi.org/10.1371/journal.pone.0077228
Xu, J., Li, L., Qian, Z., Hong, J., Shen, S., & Huang, W. (2005). Reduction of PTP1B by RNAi upregulates the activity of insulin controlled fatty acid synthase promoter. Biochemical and Biophysical Research Communications, 329(2), 538–543. https://doi.org/10.1016/J.BBRC.2005.02.016
Hussain, H., Green, I. R., Abbas, G., Adekenov, S. M., Hussain, W., & Ali, I. (2019). Protein tyrosine phosphatase 1B (PTP1B) inhibitors as potential anti-diabetes agents: Patent review (2015–2018). Expert Opinion on Therapeutic Patents, 29(9), 689–702. https://doi.org/10.1080/13543776.2019.1655542
Stringer, J. L., & Thomas, J. A. (2019). Antidiabetic drug | Description, Actions, & Uses | Britannica. Encyclopedia Britannica. Retrieved from https://www.britannica.com/science/antidiabetic-drug
Bösenberg, L. H., & Van Zyl, D. G. (2008). Journal of Endocrinology, Metabolism and Diabetes of South Africa The mechanism of action of oral antidiabetic drugs: A review of recent literature. Journal of Endocrinology Metabolism and Diabetes of South Africa, 13(3), 80–88. https://doi.org/10.1080/22201009.2008.10872177
Furman, B. L. (2011). Antidiabetic agents. In xPharm: The Comprehensive Pharmacology Reference (pp.1–1). Elsevier Inc. https://doi.org/10.1016/B978-008055232-3.61040-2
Robinson, J. (2020). Diabetes drug alternatives to insulin. WebMD. Retrieved May 13, 2021, from https://www.webmd.com/diabetes/guide/diabetes-non-insulin
Cory, H., Passarelli, S., Szeto, J., Tamez, M., & Mattei, J. (2018). The role of polyphenols in human health and food systems: A mini-review. Frontiers in Nutrition, 5, 87. https://doi.org/10.3389/fnut.2018.00087
Gotter, A. (2019). Polyphenols food list: Seasonings, berries, and more. healthline. Retrieved July 3, 2020, from https://www.healthline.com/health/polyphenols-foods
Kim, Y. A., Keogh, J. B., & Clifton, P. M. (2016). Polyphenols and glycémie control. Nutrients, 8(1). https://doi.org/10.3390/nu8010017
Solayman, M., Ali, Y., Alam, F., Asiful Islam, M., Alam, N., Khalil, I., M., & Gan, H., S (2016). Polyphenols: Potential future arsenals in the treatment of diabetes. Current Pharmaceutical Design, 22(5). https://doi.org/10.2174/1381612822666151125001111
Zakłos-Szyda, M., Majewska, I., Redzynia, M., & Koziołkiewicz, M. (2015). Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α -glucosidase and PTP1B inhibitors, and β pancreatic cells cytoprotective agents - A comparative study. Current Topics in Medicinal Chemistry, 15(23), 2431–2444. https://doi.org/10.2174/1568026615666150619143051
Umeno, A., Horie, M., Murotomi, K., Nakajima, Y., & Yoshida, Y. (2016). Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules, 21(6). https://doi.org/10.3390/molecules21060708
Grant, S. F. A., Thorleifsson, G., Reynisdottir, I., Benediktsson, R., Manolescu, A., Sainz, J., … Stefansson, K. (2006). Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes Nature Genetics, 38(3), 320–323. https://doi.org/10.1038/ng1732
Zhou, Y., Park, S.-Y., Su, J., Bailey, K., Ottosson-Laakso, E., Shcherbina, L., … Hansson, O. (2014). TCF7L2 is a master regulator of insulin production and processing. Human Molecular Genetics, 23(24), 6419–31. https://doi.org/10.1093/hmg/ddu359
Ayyanar, M., & Subash-Babu, P. (2012). Syzygium cumini (L.) Skeels: A review of its phytochemical constituents and traditional uses. Asian Pacific Journal of Tropical Biomedicine, 2(3), 240–246. https://doi.org/10.1016/S2221-1691(12)60050-1
Amin, M. M. (2020). Phyto-medicinal effects of syzygium cumini on diabetes: a review. International Journal of Research in AYUSH and Pharmaceutical Sciences, 4(3), 392–402. https://doi.org/10.47070/ijraps.v4i3.80
Ecker, A., Gonzaga, T. K. S. do N., Seeger, R. L., Santos, M. M. dos, Loreto, J. S., Boligon, A. A., … Barbosa, N. V. (2017). High-sucrose diet induces diabetic-like phenotypes and oxidative stress in Drosophila melanogaster: Protective role of Syzygium cumini and Bauhinia forficata. Biomedicine and Pharmacotherapy, 89, 605–616. https://doi.org/10.1016/j.biopha.2017.02.076
Thiyagarajan, G., Muthukumaran, P., Sarath Kumar, B., Muthusamy, V. S., & Lakshmi, B. S. (2016). Selective inhibition of PTP1B by vitalboside A from Syzygium cumini enhances insulin sensitivity and attenuates lipid accumulation via partial agonism to PPARγ: In vitro and in silico investigation. Chemical Biology and Drug Design, 88(2), 302–312. https://doi.org/10.1111/cbdd.12757
Li, L., Huang, X., & Han, L. (2017). Phloroglucinol derivative isolated from seed of Syzygium cumini capable of inhibiting activity of PTP1B. Expert Opinion on Therapeutic Patents. Northeastern University, China, assignee.
Xu, S. H., Xu, W., Wang, L., Hu, Y. K., Liu, J. P., Zhao, Y., … Zhao, Y. (2018). New phloroglucinol derivatives with protein tyrosine phosphatase 1B (PTP1B) inhibitory activities from Syzygium austroyunnanense. Fitoterapia, 131, 141–145. https://doi.org/10.1016/j.fitote.2018.10.010
Rauf, A., Khan, I. A., Muhammad, N., Al-Awthan, Y. S., Bahattab, O., Israr, M., & Mubarak, M. S. (2021). Phytochemical composition, in vitro urease, α-glucosidase and phosphodiesterase inhibitory potency of Syzygium cumini (Jamun) fruits. South African Journal of Botany. https://doi.org/10.1016/j.sajb.2021.04.006
Sharma, S. B., Nasir, A., Prabhu, K. M., Murthy, P. S., & Dev, G. (2003). Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. Journal of Ethnopharmacology, 85(2–3), 201–206. https://doi.org/10.1016/S0378-8741(02)00366-5
Kumar, A., Ilavarasan, R., Jayachandran, T., Deecaraman, M., Aravindan, P., Padmanabhan, N., & Krishan, M. R. V. (2008). Anti-diabetic activity of Syzygium cumini and it’s isolated compound against streptozotocin-induced diabetic rats Anti-diabetic activity of Syzygium cumini and its isolated compound against streptozotocin-induced diabetic rats. Journal of Medicinal Plants Research 2(9), 246–249.
Sharma, A. K., Bharti, S., Kumar, R., Krishnamurthy, B., Bhatia, J., Kumari, S., & Arya, D. S. (2012). Syzygium cumini ameliorates insulin resistance and β-cell dysfunction via modulation of PPARγ, dyslipidemia, oxidative stress, and TNF-α in type 2 diabetic rats. Journal of Pharmacological Sciences, 119(3), 203–213. https://doi.org/10.1254/jphs.11184FP
Syama, H. P., Arya, A. D., Dhanya, R., Nisha, P., Sundaresan, A., Jacob, E., & Jayamurthy, P. (2017). Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. Journal of Food Science and Technology, 54(7), 2115–2125. https://doi.org/10.1007/s13197-017-2651-3
Ulla, A., Alam, M. A., Sikder, B., Sumi, F. A., Rahman, M. M., Habib, Z. F., … Reza, H. M. (2017). Supplementation of Syzygium cumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complementary and Alternative Medicine, 17(1), 1–13. https://doi.org/10.1186/s12906-017-1799-8
Sagor, A. T., Mohib, M., Tabassum, N., Ahmed, I., Reza, M., & Alam, M. A. (2016). Fresh seed supplementation of Syzygium cumini attenuated oxidative stress, inflammation, fibrosis, iron overload, hepatic dysfunction and renal injury in acetaminophen induced rats. https://doi.org/10.4172/2157-7609.1000208
Mohammadi, J., & Naik, P. (2012). The histopathologic effects of Morus alba leaf extract on the pancreas of diabetic rats. Turkish Journal of Biology, 36, 211–216. https://doi.org/10.3906/biy-1008-51
Hansawasdi, C., & Kawabata, J. (2006). α-Glucosidase inhibitory effect of mulberry (Morus alba) leaves on Caco-2. Fitoterapia, 77(7–8), 568–573. https://doi.org/10.1016/j.fitote.2006.09.003
Niu, S. L., Tong, Z. F., Zhang, Y., Liu, T. L., Tian, C. L., Zhang, D. X., … Tian, J. L. (2020). Novel protein tyrosine phosphatase 1B inhibitor-geranylated flavonoid from mulberry leaves ameliorates insulin resistance. Journal of Agricultural and Food Chemistry, 68(31), 8223–8231. https://doi.org/10.1021/acs.jafc.0c02720
Singab, A. N. B., El-Beshbishy, H. A., Yonekawa, M., Nomura, T., & Fukai, T. (2005). Hypoglycemic effect of Egyptian Morus alba root bark extract: Effect on diabetes and lipid peroxidation of streptozotocin-induced diabetic rats. Journal of Ethnopharmacology, 100(3), 333–338. https://doi.org/10.1016/j.jep.2005.03.013
Platel, K., & Srinivasan, K. (1997). Plant foods in the management of Diabetes mellitus: Vegetables as potential hypoglycaemic agents. Food / Nahrung, 41(2), 68–74. https://doi.org/10.1002/food.19970410203
Chen, F., Nakashima, N., Kimura, I., & Kimura, M. (1995). Hypoglycemic activity and mechanisms of extracts from Mulberry leaves (Folium Mori) and Cortex Mori Radicis in streptozotocin-induced diabetic mice. Yakugaku Zasshi, 115(6), 476–482. https://doi.org/10.1248/yakushi1947.115.6_476
Ha, M. T., Shrestha, S., Tran, T. H., Kim, J. A., Woo, M. H., Choi, J. S., & Min, B. S. (2020). Inhibition of PTP1B by farnesylated 2-arylbenzofurans isolated from Morus alba root bark: Unraveling the mechanism of inhibition based on in vitro and in silico studies. Archives of Pharmacal Research, 43(9), 961–975. https://doi.org/10.1007/s12272-020-01269-4
Paudel, P., Yu, T., Seong, S. H., Kuk, E. B., Jung, H. A., & Choi, J. S. (2018). Protein tyrosine phosphatase 1B inhibition and glucose uptake potentials of mulberrofuran G, albanol B, and kuwanon G from root bark of Morus alba L. in insulin-resistant HepG2 cells: An in vitro and in silico study. International Journal of Molecular Sciences, 19(5), 1542. https://doi.org/10.3390/ijms19051542
Choi, K. H., Lee, H. A., Park, M. H., & Han, J. S. (2016). Mulberry (Morus alba L.) fruit extract containing anthocyanins improves glycemic control and insulin sensitivity via activation of AMP-activated protein kinase in diabetic C57BL/Ksj-db/db mice. Journal of Medicinal Food, 19(8), 737–745. https://doi.org/10.1089/jmf.2016.3665
Meng, Q., Qi, X., Fu, Y., Chen, Q., Cheng, P., Yu, X., … Bian, H. (2020). Flavonoids extracted from mulberry (Morus alba L.) leaf improve skeletal muscle mitochondrial function by activating AMPK in type 2 diabetes. Journal of Ethnopharmacology, 248. https://doi.org/10.1016/j.jep.2019.112326
Yan, F., Dai, G., & Zheng, X. (2016). Mulberry anthocyanin extract ameliorates insulin resistance by regulating PI3K/AKT pathway in HepG2 cells and db/db mice. Journal of Nutritional Biochemistry, 36, 68–80. https://doi.org/10.1016/j.jnutbio.2016.07.004
Lim, S. H., Yu, J. S., Lee, H. S., Choi, C. I., & Kim, K. H. (2021). Antidiabetic flavonoids from fruits of Morus alba promoting insulin-stimulated glucose uptake via Akt and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Pharmaceutics, 13(4), 526. https://doi.org/10.3390/pharmaceutics13040526
Kimura, T., (National, A. R. C., & for, T. R. J. (2011). Development of mulberry leaf extract for suppressing postprandial blood glucose elevation. InTech. Retrieved from https://cdn.intechopen.com/pdfs/21464/InTech-Development_of_mulberry_leaf_extract_for_suppressing_postprandial_blood_glucose_elevation.pdf. Accessed 10 Aug 2021.
El-Beshbishy, H. A., Singab, A. N. B., Sinkkonen, J., & Pihlaja, K. (2006). Hypolipidemic and antioxidant effects of Morus alba L. (Egyptian mulberry) root bark fractions supplementation in cholesterol-fed rats. Life Sciences, 78(23), 2724–2733. https://doi.org/10.1016/j.lfs.2005.10.010
Hussain, F., Rana, Z., Shafique, H., Malik, A., & Hussain, Z. (2017). Phytopharmacological potential of different species of Morus alba and their bioactive phytochemicals: A review. Asian Pacific Journal of Tropical Biomedicine. Hainan Medical University. https://doi.org/10.1016/j.apjtb.2017.09.015
Vengerovskii, A. I., Khazanov, V. A., Eskina, K. A., & Vasilyev, K. Y. (2007). Effects of silymarin (hepatoprotector) and succinic acid (bioenergy regulator) on metabolic disorders in experimental diabetes mellitus. Bulletin of Experimental Biology and Medicine, 144(1), 53–56. https://doi.org/10.1007/s10517-007-0252-2
Tajmohammadi, A., Razavi, B. M., & Hosseinzadeh, H. (2018). Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: A review. Phytotherapy Research, 32(10), 1933–1949. https://doi.org/10.1002/ptr.6153
Alhusban, A., Alkhazaleh, E., & El-Elimat, T. (2017). Silymarin ameliorates diabetes-induced proangiogenic response in brain endothelial cells through a GSK-3β inhibition-induced reduction of VEGF release. https://doi.org/10.1155/2017/2537216
Meng, S., Yang, F., Wang, Y., Qin, Y., **an, H., Che, H., & Wang, L. (2019). Silymarin ameliorates diabetic cardiomyopathy via inhibiting TGF-β1/Smad signaling. Cell Biology International, 43(1), 65–72. https://doi.org/10.1002/cbin.11079
Qin, N., Hu, X., Li, S., Wang, J., Li, Z., Li, D., … Hua, H. (2017). Hypoglycemic effect of silychristin A from Silybum marianum fruit via protecting pancreatic islet β cells from oxidative damage and inhibiting α-glucosidase activity in vitro and in rats with type 1 diabetes. Journal of Functional Foods, 38, 168–179. https://doi.org/10.1016/j.jff.2017.09.013
Cheng, B., Gong, H., Li, X., Sun, Y., Zhang, X., Chen, H., … Huang, K. (2012). Silibinin inhibits the toxic aggregation of human islet amyloid polypeptide. Biochemical and Biophysical Research Communications, 419(3), 495–499. https://doi.org/10.1016/j.bbrc.2012.02.042
Detaille, D., Sanchez, C., Sanz, N., Lopez-Novoa, J. M., Leverve, X., & El-Mir, M. Y. (2008). Interrelation between the inhibition of glycolytic flux by silibinin and the lowering of mitochondrial ROS production in perifused rat hepatocytes. Life Sciences, 82(21–22), 1070–1076. https://doi.org/10.1016/j.lfs.2008.03.007
Palomino, O. M., Gouveia, N. M., Ramos, S., Martín, M. A., & Goya, L. (2017). Protective effect of Silybum marianum and silibinin on endothelial cells submitted to high glucose concentration. Planta Medica, 83(1–2), 97–103. https://doi.org/10.1055/s-0042-113135
Das, S., Roy, P., Pal, R., Auddy, R. G., Chakraborti, A. S., & Mukherjee, A. (2014). Engineered silybin nanoparticles educe efficient control in experimental diabetes. PLoS One1, 9(7), 101818. https://doi.org/10.1371/journal.pone.0101818
Toğay, V. A., Sevimli, T. S., Sevimli, M., Çelik, D. A., & Özçelik, N. (2018). DNA damage in rats with streptozotocin-induced diabetes; Protective effect of silibinin. Mutation Research - Genetic Toxicology and Environmental Mutagenesis, 825, 15–18. https://doi.org/10.1016/j.mrgentox.2017.11.002
Qin, N., Sasaki, T., Li, W., Wang, J., Zhang, X., Li, D., … Koike, K. (2018). Identification of flavonolignans from Silybum marianum seeds as allosteric protein tyrosine phosphatase 1B inhibitors. Journal of Enzyme Inhibition and Medicinal Chemistry, 33(1), 1283–1291. https://doi.org/10.1080/14756366.2018.1497020
Qin, N. bo, Jia, C. cui, Xu, J., Li, D. hong, Xu, F. xing, Bai, J., … Hua, H. ming. (2017). New amides from seeds of Silybum marianum with potential antioxidant and antidiabetic activities. Fitoterapia, 119, 83–89. https://doi.org/10.1016/j.fitote.2017.04.008
Murotomi, K., Umeno, A., Yasunaga, M., Shichiri, M., Ishida, N., Koike, T., … Nakajima, Y. (2015). Oleuropein-Rich Diet Attenuates Hyperglycemia and Impaired Glucose Tolerance in Type 2 Diabetes Model Mouse. Journal of Agricultural and Food Chemistry, 63(30), 6715–6722. https://doi.org/10.1021/acs.jafc.5b00556
Pérez-Jiménez, A., Rufino-Palomares, E. E., Fernández-Gallego, N., Ortuño-Costela, M. C., Reyes-Zurita, F. J., Peragón, J., … Lupiáñez, J. A. (2016). Target molecules in 3T3-L1 adipocytes differentiation are regulated by maslinic acid, a natural triterpene from Olea europaea. Phytomedicine, 23(12), 1301–1311. https://doi.org/10.1016/j.phymed.2016.07.001
Hadrich, F., Garcia, M., Maalej, A., Moldes, M., Isoda, H., Feve, B., & Sayadi, S. (2016). Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sciences, 151, 167–173. https://doi.org/10.1016/j.lfs.2016.02.027
Hardie, D. G. (2011). September 15). AMP-activated protein kinase-an energy sensor that regulates all aspects of cell function. Genes and Development. Cold Spring Harbor Laboratory Press. https://doi.org/10.1101/gad.17420111
Wolfson Medical Center (2011). Effect of Olive Leaves as Hypoglycemic Agents in Diabetic Subjects - Full Text View - ClinicalTrials.gov. Retrieved April 18, 2019, from https://clinicaltrials.gov/ct2/show/NCT01427998
Wainstein, J., Ganz, T., Boaz, M., Dayan, B., Dolev, Y., Kerem, E., & Madar, Z. (2012). Olive leaf extract as a hypoglycemic agent in both human diabetic subjects and in rats. Journal of Medicinal Food, 15(7), 605–610. https://doi.org/10.1089/jmf.2011.0243
Hansen, S. K., Cancilla, M. T., Shiau, T. P., Kung, J., Chen, T., & Erlanson, D. A. (2005). Allosteric inhibition of PTP1B activity by selective modification of a non-active site cysteine residue † Biochemistry, 44(21), 7704–7712. https://doi.org/10.1021/bi047417s
Jiang, C. S., Liang, L. F., & Guo, Y. W. (2012, October). Natural products possessing protein tyrosine phosphatase 1B (PTP1B) inhibitory activity found in the last decades. Acta Pharmacologica Sinica Nature Publishing Group. https://doi.org/10.1038/aps.2012.90
Alkhatib, A., Tsang, C., & Tuomilehto, J. (2018, July 12). Olive oil nutraceuticals in the prevention and management of diabetes: From molecules to lifestyle. International Journal of Molecular Sciences. MDPI AG. https://doi.org/10.3390/ijms19072024
Li, F., Li, Q., Shi, X., & Guo, Y. (2017). Maslinic acid inhibits impairment of endothelial functions induced by high glucose in HAEC cells through improving insulin signaling and oxidative stress. Biomedicine and Pharmacotherapy, 95, 904–913. https://doi.org/10.1016/j.biopha.2017.09.001
Lakshmi, B. S., Sujatha, S., Anand, S., Sangeetha, K. N., Narayanan, R. B., Katiyar, C., … Singh, S. (2009). Cinnamic acid, from the bark of Cinnamomum cassia, regulates glucose transport via activation of GLUT4 on L6 myotubes in a phosphatidylinositol 3-kinase-independent manner. Journal of diabetes, 1(2), 99–106. https://doi.org/10.1111/j.1753-0407.2009.00022.x
Lin, G. M., Chen, Y. H., Yen, P. L., & Chang, S. T. (2016). Antihyperglycemic and antioxidant activities of twig extract from Cinnamomum osmophloeum. Journal of Traditional and Complementary Medicine, 6(3), 281–288. https://doi.org/10.1016/j.jtcme.2015.08.005
Lin, G. M., Lin, H. Y., Hsu, C. Y., & Chang, S. T. (2016). Structural characterization and bioactivity of proanthocyanidins from indigenous cinnamon (Cinnamomum osmophloeum). Journal of the Science of Food and Agriculture, 96(14), 4749–4759. https://doi.org/10.1002/jsfa.7802
Gaber, E., & El-Desoky. (2012). Antidiabetic and hypolipidemic effects of Ceylon cinnamon (Cinnamomum verum) in alloxan-diabetic rats. Journal of Medicinal Plants Research, 6(9), 1685–1691. https://doi.org/10.5897/jmpr11.1472
Mang, B., Wolters, M., Schmitt, B., Kelb, K., Lichtinghagen, R., Stichtenoth, D. O., & Hahn, A. (2006). Effects of a cinnamon extract on plasma glucose, HbA1c, and serum lipids in diabetes mellitus type 2. European Journal of Clinical Investigation, 36(5), 340–344. https://doi.org/10.1111/j.1365-2362.2006.01629.x
Anderson, R. A. (2008). Chromium and polyphenols from cinnamon improve insulin sensitivity. Proceedings of the Nutrition Society, 67(01), 48–53. https://doi.org/10.1017/S0029665108006010
Khan, A., Safdar, M., Ali Khan, M. M., Khattak, K. N., & Anderson, R. A. (2003). Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care, 26(12), 3215–3218. https://doi.org/10.2337/diacare.26.12.3215
Muhammad, S., Sangi, A., Fawzy, M., & El-Wahab, A. (2017). Experimental evaluations of the nephroprotective properties of ginger (Zingiber officinale), Cinnamomum verum and Nigella sativa in STZ induced diabetic rats. IJBPAS, 6(6), 1195–1209. Retrieved from https://www.researchgate.net/publication/317412411. Accessed 18 Aug 2021.
Zare, R., Nadjarzadeh, A., Zarshenas, M. M., Shams, M., & Heydari, M. (2019). Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clinical Nutrition, 38(2), 549–556. https://doi.org/10.1016/j.clnu.2018.03.003
Verspohl, E. J., Bauer, K., & Neddermann, E. (2005). Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytotherapy Research, 19(3), 203–206. https://doi.org/10.1002/ptr.1643
Lamuchi-Deli, N., Aberomand, M., Babaahmadi-Rezaei, H., & Mohammadzadeh, G. (2017). Effects of the hydroalcoholic extract of Zingiber officinale on arginase i activity and expression in the retina of streptozotocin-induced diabetic rats. International Journal of Endocrinology and Metabolism, 15(2), 42161. https://doi.org/10.5812/ijem.42161
Akhani, S. P., Vishwakarma, S. L., & Goyal, R. K. (2004). Anti-diabetic activity of Zingiber officinale in streptozotocin-induced type I diabetic rats. Journal of Pharmacy and Pharmacology, 56(1), 101–105. https://doi.org/10.1211/0022357022403
Dodda, H., Ramadas, D., Mundasada, S. C., Nune, S. K., Lingaraju, S. K., & Puttaswamygowda, R. (2016). Anti-diabetic activity of ginger tuber proteins: In vitro studies. Asian Journal of Pharmaceutical and Health Sciences, 6(2), 1–5.
Hester, F., Verghese, M., Sunkara, R., Willis, S., & Walker, L. T. (2019). A comparison of the antioxidative and anti-diabetic potential of thermally treated garlic, turmeric, and ginger. Food and Nutrition Sciences, 10(02), 207–219. https://doi.org/10.4236/fns.2019.102016
Abdi, T., Mahmoudabady, M., Marzouni, H. Z., Niazmand, S., & Khazaei, M. (2020). Ginger (Zingiber Officinale Roscoe) extract protects heart against inflammation and fibrosis in diabetic rats. Canadian Journal of Diabetes. https://doi.org/10.1016/j.jcjd.2020.08.102
Lamba, S. S., Buch, K. Y., Lewis, H., & Lamba, J. (2000). Phytochemicals as potential hypoglycemic agents. Studies in Natural Products Chemistry, 21, 457–496. https://doi.org/10.1016/S1572-5995(00)80012-5
Lakshmi, T., Ramasamy, R., & Thirumalaikumaran, R. (2015). Preliminary phytochemical analysis and in vitro antioxidant, FTIR spectroscopy, anti-diabetic activity of Acacia catechu ethanolic seed extract. Pharmacognosy Journal, 7(6). https://doi.org/10.5530/pj.2015.6.7
Ikarashi, N., Toda, T., Okaniwa, T., Ito, K., Ochiai, W., & Sugiyama, K. (2011). Anti-obesity and anti-diabetic effects of acacia polyphenol in obese diabetic KKAy mice fed high-fat diet, 2011. https://doi.org/10.1093/ecam/nep241
Choudhary, N., Khatik, G. L., & Suttee, A. (2020). The possible role of saponin in type-II diabetes- A review. Current Diabetes Reviews, 17(2), 107–121. https://doi.org/10.2174/1573399816666200516173829
Mohammad-Alizadeh, S., Javadzadeh, Y., & Mirghafourvand, M. (2018). Effects of garlic pill on blood glucose level in borderline gestational diabetes mellitus: A randomized controlled trial Pharmaceutical Nanotechnology View project Comaprison of menopausal symptoms and quality of life between urban and rural middle age women View project 136 PUBLICATIONS 2,490 CITATIONS SEE PROFILE. Iranian Red Crescent Medical Journal. https://doi.org/10.5812/ircmj.60675
Batiha, G. E. S., Beshbishy, A. M., Wasef, L. G., Elewa, Y. H. A., Al-Sagan, A. A., El-Hack, M. E. A., … Devkota, H. P. (2020). Chemical constituents and pharmacological activities of garlic (Allium sativum L.): A review. Nutrients, 12(3), 872. https://doi.org/10.3390/nu12030872
Velsankar, K., Preethi, R., Ram, P. S. J., Ramesh, M., & Sudhahar, S. (2020). Evaluations of biosynthesized Ag nanoparticles via Allium sativum flower extract in biological applications. Applied Nanoscience (Switzerland), 10(9), 3675–3691. https://doi.org/10.1007/s13204-020-01463-2
Nawaz, H., Shad, M. A., & Muzaffar, S. (2018). Phytochemical composition and antioxidant potential of Brassica. In Brassica Germplasm - Characterization, Breeding and Utilization. InTech. https://doi.org/10.5772/intechopen.76120
Anand, P., Murali, Y. K., Tandon, V., Murthy, P. S., & Chandra, R. (2009). Insulinotropic effect of aqueous extract of Brassica nigra improves glucose homeostasis in streptozotocin induced diabetic rats. Experimental and Clinical Endocrinology and Diabetes, 117(6), 251–256. https://doi.org/10.1055/s-2008-1080917
Anand, P., Murali, K. Y., Tandon, V., Chandra, R., & Murthy, P. S. (2007). Preliminary studies on antihyperglycemic effect of aqueous extract of Brassica nigra (L.) Koch in streptozotocin induced diabetic rats. Indian Journal of Experimental Biology, 45(8), 696–701. Retrieved from https://pubmed.ncbi.nlm.nih.gov/17877146/. Accessed 04 May 2021.
Thirumalai, T., Therasa, S. V., Elumalai, E. K., & David, E. (2011). Hypoglycemic effect of Brassica juncea (seeds) on streptozotocin induced diabetic male albino rat. Asian Pacific Journal of Tropical Biomedicine, 1(4), 323–325. https://doi.org/10.1016/S2221-1691(11)60052-X
Saloko, S., Resti Setyaningrum, D., Asih Purwestri, Y., Endro Nugroho, A., Pranoto, Y., & Widyastuti, S. (2020). α-Amilase inhibitory activity of fraction of ethanolic extract of lebui seed (Cajanus cajan (L.) Millsp.) grown in West Nusa Tenggara. Journal of Food and Pharmaceutical Sciences, 8(2), 268–274. Retrieved from www.journal.ugm.ac.id/v3/JFPS. Accessed 05 May 2021.
Igboabuchi, N. A. (2021). A comparative phytochemical and nutritional study on Cajanus cajan (L.) Millspaugh and Vigna unguiculata (L.) Walp (Fabaceae). Asian Journal of Research in Botany, 5(2), 53–59. Retrieved from https://journalajrib.com/index.php/AJRIB/article/view/30143/56571. Accessed 05 May 2021.
Ezike, A. C., Akah, P. A., Okoli, C. C., & Okpala, C. B. (2010). Experimental evidence for the antidiabetic activity of Cajanus cajan leaves in rats. Journal of Basic and Clinical Pharmacy, 1(2), 81–4. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24825970. Accessed 05 May 2021.
Mechchate, H., Es-Safi, I., Amaghnouje, A., Boukhira, S., Alotaibi, A. A., Al-Zharani, M., … Bousta, D. (2021). Antioxidant, anti-inflammatory and antidiabetic proprieties of LC-MS/MS identified polyphenols from coriander seeds. Molecules, 26(2), 487. https://doi.org/10.3390/molecules26020487
Nhut, P. T., Quyen, T. N., Truc, T. T., Minh, L. V., An, T. N. T., & Anh, N. H. T. (2020). Preliminary study on phytochemical, phenolic content, flavonoids and antioxidant activity of Coriandrum sativum l. originating in Vietnam. IOP Conference Series: Materials Science and Engineering. https://doi.org/10.1088/1757-899X/991/1/012022
Muhammad, D. R. A., Tuenter, E., Patria, G. D., Foubert, K., Pieters, L., & Dewettinck, K. (2021). Phytochemical composition and antioxidant activity of Cinnamomum burmannii Blume extracts and their potential application in white chocolate. Food Chemistry, 340, 127983. https://doi.org/10.1016/j.foodchem.2020.127983
Verdini, L., Setiawan, B., Sinaga, T., Sulaeman, A., Wayan, I., & Wibawan, T. (2020). Phytochemical profile of cinnamon extract (Cinnamomum Burmanii Blume) from three regions of sumatra island using GCMS. European Journal of Molecular & Clinical Medicine, 7(2).
Singh, N., Rao, A. S., Nandal, A., Kumar, S., Yadav, S. S., Ganaie, S. A., & Narasimhan, B. (2021). Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chemistry, 338, 127773. https://doi.org/10.1016/j.foodchem.2020.127773
Augustine, M., Saravanan, R., Atchitha, S. S., Santhiya, K., Rithika, M., Menaka, S. S., & Thiruvalluvan, T. (2020). Effect of Gymnema sylvestre leaf extract on Streptozotocin induced diabetic rats. A single stage high gain converter for grid interconnected renewable application using perturb and observe view project. Journal of Pharmacognosy and Phytochemistry. https://doi.org/10.22271/phyto.2020.v9.i4a.11663
Ghous, T., Akhtar, K., Andleeb, S., Khizar, S., Ali, S., Mustafa, R. G., … Naseer, A. (2021). Evaluation of α-glucosidase inhibition, antioxidant and antibacterial effects of Gymnema sylvestre R. Br. Bangladesh Journal of Botany, 50(1), 61–68. https://doi.org/10.3329/bjb.v50i1.52672
Venugopal, D., & Dhanasekaran, S. (2021). Bitter gourd (Momordica charantia) as an emerging therapeutic agent: Modulating metabolic regulation and cell signaling cascade. In Studies in Natural Products Chemistry (Vol.67, pp.221–268). Elsevier B.V. https://doi.org/10.1016/B978-0-12-819483-6.00007-2
Nigam, R., Sen, P., Singh, R., & Garg, P. (2021). Comparative phytochemical screening of Karela (Momordica Charantia) and Jambul (Syzygium cumini) claimed for treatment of diabetes mellitus. The Journal of Phytopharmacology, 10(1), 22–25. https://doi.org/10.31254/phyto.2021.10106
Li, Z., **a, A., Li, S., Yang, G., **, W., Zhang, M., & Wang, S. (2020). The pharmacological properties and therapeutic use of bitter melon (Momordica charantia L.). Current Pharmacology Reports, 6(3), 103–109. https://doi.org/10.1007/s40495-020-00219-4
Chokki, M., Cudalbeanu, M., Zongo, C., Dah-Nouvlessounon, D., Ghinea, I. O., Furdui, B., … Baba-Moussa, F. (2020). Exploring antioxidant and enzymes (A-Amylase and B-Glucosidase) inhibitory activity of Morinda lucida and Momordica charantia leaves from benin. Foods, 9(4), 434. https://doi.org/10.3390/foods9040434
Abd Ghafar, S. Z., Mediani, A., Maulidiani, M., Rudiyanto, R., Ghazali, M., Ramli, H., & Abas, F. (2020). Complementary NMR- and MS-based metabolomics approaches reveal the correlations of phytochemicals and biological activities in Phyllanthus acidus leaf extracts. Food Research International, 136, 109312. https://doi.org/10.1016/j.foodres.2020.109312
Ajayi, G. O., Olorunrinu, T. J., & Shittu, M. A. (2020). Elucidation of bioactive compounds in hydroalcohol extract of Phyllanthus amarus Schum. and Thonn. leaf using GC-MS analysis. Journal of Scientific and Innovative Research, 9(2), 40–47. Retrieved from https://www.jsirjournal.com. Accessed 05 May 2021.
Harikrishnan, H., Jantan, I., Alagan, A., & Haque, M. A. (2020). Modulation of cell signaling pathways by Phyllanthus amarus and its major constituents: Potential role in the prevention and treatment of inflammation and cancer. Inflammopharmacology. Springer. https://doi.org/10.1007/s10787-019-00671-9
Sharma, P., Joshi, T., Joshi, T., Chandra, S., & Tamta, S. (2020). Molecular dynamics simulation for screening phytochemicals as α-amylase inhibitors from medicinal plants. Journal of Biomolecular Structure and Dynamics. https://doi.org/10.1080/07391102.2020.1801507
Mandar, B. K., Khanal, P., Patil, B. M., Dey, Y. N., & Pasha, I. (2021). In silico analysis of phytoconstituents from Tinospora cordifolia with targets related to diabetes and obesity. In Silico Pharmacology, 9(1), 1–9. https://doi.org/10.1007/s40203-020-00063-w
Shridhar, N., Shesharao, S., & Ml, S. N. (2020). Phytochemical analysis of Trigonella foenum graecum and Coccinia indica for their different components by HPTLC. Pharma Innovation, 9(9), 52-57. Retrieved from http://www.thepharmajournal.com. Accessed 05 May 2021.
Z, B., & F, A. (2015). Dietary phytochemical index and the risk of insulin resistance and β-cell dysfunction: A prospective approach in Tehran lipid and glucose study. International Journal of Food Sciences and Nutrition, 66(8), 950–955. https://doi.org/10.3109/09637486.2015.1111867
Acknowledgements
The authors are grateful to the founder president of Amity University Dr. Ashok K. Chauhan for motivating, providing all facilities, and constant support. The authors acknowledge the Council of Scientific and Industrial Research (CSIR) India for fellowship support [09/915(0017)/2019-EMR-I].
Author information
Authors and Affiliations
Contributions
P.R, T.J, A.R., A.C, R.P.: conceptualisation; P.R: writing and editing; T.J, A.R, A.C, N.K.V, A.B., R.P,: reviewing; T.J: resources. All the authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals.
Consent to Participate
All authors agree mutually with the participation and publication of this work and declare that this is original research.
Consent to Publish
All authors agree mutually to publication of this work.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rath, P., Ranjan, A., Chauhan, A. et al. A Critical Review on Role of Available Synthetic Drugs and Phytochemicals in Insulin Resistance Treatment by Targeting PTP1B. Appl Biochem Biotechnol 194, 4683–4701 (2022). https://doi.org/10.1007/s12010-022-04028-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04028-x