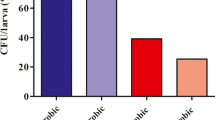
Abstract
This study aimed to detect and identify the N-acyl-homoserine lactones molecules (AHLs) produced by different resistant Klebsiella pneumoniae isolates recovered from poultry and environmental samples using a modified validated high-performance liquid chromatography method. A total of 56 K. pneumoniae isolates were recovered, investigated for their antibiotic susceptibility, and screened for AHLs production using the Agrobacterium tumefaciens NTL4 biosensor system and a validated high-performance liquid chromatography method. The results revealed the detection of different short- and long-chain AHLs molecules among 39 K. pneumoniae isolates recovered from poultry and environmental samples. All environmental isolates produced nine peaks with retention times for C4-HSL, C6-HSL, C12-HSL, C8-HSL, C14-HSL, C8-oxo-HSL, C10-HSL, C6-oxo-HSL, and C7-HSL. The most quantifiable AHL signal molecules in poultry isolates were C4-HSL, C6-HSL, and C12-HSL. No statistical correlation between the AHL-producing ability of K. pneumoniae isolates and antibiotic resistance was reported. To the best of our knowledge, this study provides the first detailed report on the detection and identification of AHLs in K. pneumoniae isolates recovered from poultry and environmental samples. Furthermore, it provides a new insight available tool other than LC-MS/MS for detection and identification of AHL molecules.


Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its supporting information.
References
Hayati, M., Indrawati, A., Mayasari, N. L. P. I., Istiyaningsih, I., & Atikah, N. (2019). Molecular detection of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates of chicken origin from East Java, Indonesia. Veterinary World, 12(4), 578–583.
Guo, Y., Zhou, H., Qin, L., Pang, Z., Qin, T., Ren, H., & Zhou, J. (2016). Frequency, antimicrobial resistance and genetic diversity of Klebsiella pneumoniae in food samples. PLoS One, 11(4), e0153561.
Savin, M., Bierbaum, G., Hammerl, J. A., Heinemann, C., Parcina, M., Sib, E., & Kreyenschmidt, J. (2020). ESKAPE bacteria and extended-spectrum-β-lactamase-producing Escherichia coli isolated from wastewater and process water from German poultry slaughterhouses. Applied and Environmental Microbiology, 86(8).
Chen, L., Wilksch, J. J., Liu, H., Zhang, X., Torres, V. V., Bi, W., ... & Zhou, T. (2020). Investigation of LuxS-mediated quorum sensing in Klebsiella pneumoniae. Journal of Medical Microbiology, 69(3), 402, 413.
Koraimann, G., & Wagner, M. A. (2014). Social behavior and decision making in bacterial conjugation. Frontiers in Cellular and Infection Microbiology, 4, 54.
Liu, W., Lu, H., Chu, X., Lou, T., Zhang, N., Zhang, B., & Chu, W. (2020). Tea polyphenols inhibits biofilm formation, attenuates the quorum sensing-controlled virulence and enhances resistance to Klebsiella pneumoniae infection in Caenorhabditis elegans model. Microbial Pathogenesis, 147, 104266.
Yin, W.-F., Purmal, K., Chin, S., Chan, X.-Y., Koh, C.-L., Sam, C.-K., & Chan, K.-G. (2012). N-acyl homoserine lactone production by Klebsiella pneumoniae isolated from human tongue surface. Sensors, 12(3), 3472–3483.
Nievas, F., Bogino, P., Sorroche, F., & Giordano, W. (2012). Detection, characterization, and biological effect of quorum-sensing signaling molecules in peanut-nodulating bradyrhizobia. Sensors, 12(3), 2851–2873.
Ngeow, Y. F., Cheng, H. J., Chen, J. W., Yin, W.-F., & Chan, K.-G. (2013). Short chain N-acylhomoserine lactone production by clinical multidrug resistant Klebsiella pneumoniae strain CSG20. Sensors, 13(11), 15242–15251.
Mukesh, K., Harjai, K., & Chhibber, S. (2017). Prevalence of AI-1 type of quorum sensing molecules in Klebsiella pneumoniae clinical isolates. International Journal of Advanced Research, 5(7), 101–107.
Gui, M., Liu, L., Wu, R., Hu, J., Wang, S., & Li, P. (2018). Detection of new quorum sensing N-acyl homoserine lactones from Aeromonas veronii. Frontiers in Microbiology, 9, 1712.
Steindler, L., & Venturi, V. (2007). Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiology Letters, 266(1), 1–9.
Hamza, E., Dorgham, S. M., & Hamza, D. A. (2016). Carbapenemase-producing Klebsiella pneumoniae in broiler poultry farming in Egypt. Journal of Global Antimicrobial Resistance, 7, 8–10.
Hansen, D. S., Aucken, H. M., Abiola, T., & Podschun, R. (2004). Recommended test panel for differentiation of Klebsiella species on the basis of a trilateral interlaboratory evaluation of 18 biochemical tests. Journal of Clinical Microbiology, 42(8), 3665–3669.
CLSI. (2017). Performance standards for antimicrobial susceptibility testing: Twenty-Seventh Informational Supplement. CLSI Document M100-S27. Clinical and Laboratory Standards Institute Wayne, PA.
Lade, H., Paul, D., & Kweon, J. H. (2014). Isolation and molecular characterization of biofouling bacteria and profiling of quorum sensing signal molecules from membrane bioreactor activated sludge. International Journal of Molecular Sciences, 15(2), 2255–2273.
ICH Q2(R1) (2005). Validation of analytical procedures: text and methodology. International Conference on Harmonization, Geneva.
USP (2017). US Pharmacopeia National Formulary 2017: USP 27 NF 22. In (Vol. 2, pp. 2281). United States Pharmacopeial Convention, Rockville.
Yang, Y., Zhou, M., Hardwidge, P. R., Cui, H., & Zhu, G. (2018). Isolation and characterization of N-acyl homoserine lactone-producing bacteria from cattle rumen and swine intestines. Frontiers in Cellular Infection Microbiology, 8, 155.
Threlfall, E. J., Wain, J., Peters, T., Lane, C., De Pinna, E., Little, C. L., Wales, A. D., & Davies, R. H. (2014). Egg-borne infections of humans with Salmonella: not only an S. Enteritidis problem. Worlds Poultry Science Journal, 70(1), 15–26.
CDC. (2015). Centers for Disease Control National Antimicrobial Resistance Monitoring System for Enteric Bacteria (NARMS): the 2013 NARMS annual human isolates report. US Department of Health Human Services, CDC, Atlanta.
Gantois, I., Ducatelle, R., Pasmans, F., Haesebrouck, F., Gast, R., Humphrey, T. J., & Van Immerseel, F. (2009). Mechanisms of egg contamination by Salmonella Enteritidis. FEMS Microbiology Reviews, 33(4), 718–738.
Khan, K. A., Khan, S. A., Aslam, A., Rabbani, M., & Tipu, M. Y. (2004). Factors contributing to yolk retention in poultry: a review. Pakistan Veterinary Journal, 24, 46–51.
Rehault-Godbert, S., Baron, F., Mignon-Grasteau, S., Labas, V., Gautier, M., Hincke, M. T., & Nys, Y. (2010). Effect of temperature and time of storage on protein stability and anti-Salmonella activity of egg white. Journal Of Food Protection, 73(9), 1604–1612.
Wyres, K. L., & Holt, K. E. (2018). Klebsiella pneumoniae as a key trafficker of drug resistance genes from environmental to clinically important bacteria. Current Opinion in Microbiology, 45, 131–139.
Struve, C., & Krogfelt, K. A. (2004). Pathogenic potential of environmental Klebsiella pneumoniae isolates. Environmental Microbiology, 6(6), 584–590.
Khelfa, D., & Morsy, E. A. (2015). Incidence and distribution of some aerobic bacterial agents associated with high chick mortality in some broiler flocks in Egypt. Middle East Journal of Applied Sciences, 5, 383–394.
Aly, M. M., Khalil, S., & Metwaly, A. (2014). Isolation and molecular identification of Klebsiella microbe isolated from chicks. Alexandria Journal for Veterinary Sciences, 43(1), 97–103.
Ojo, O. E., Ogunyinka, O. G., Agbaje, M., Okuboye, J. O., Kehinde, O. O., & Oyekunle, M. A. (2012). Antibiogram of Enterobacteriaceae isolated from free-range chickens in Abeokuta, Nigeria. Veterinarski Arhiv, 82(6), 577–589.
Ebomah, K. E., & Okoh, A. I. (2020). Detection of carbapenem-resistance genes in Klebsiella species recovered from selected environmental niches in the Eastern Cape Province, South Africa. Antibiotics, 9(7), 425.
Samanta, A., Mahanti, A., Chatterjee, S., Joardar, S. N., Bandyopadhyay, S., Sar, T. K., & Samanta, I. (2018). Pig farm environment as a source of beta-lactamase or AmpC-producing Klebsiella pneumoniae and Escherichia coli. Annals of Microbiology, 68(11), 781–791.
Wu, H., Wang, M., Liu, Y., Wang, X., Wang, Y., Lu, J., & Xu, H. (2016). Characterization of antimicrobial resistance in Klebsiella species isolated from chicken broilers. International Journal of Food Microbiology, 232, 95–102.
Kim, S.-H., Wei, C.-I., Tzou, Y.-M., & An, H. (2005). Multidrug-resistant Klebsiella pneumoniae isolated from farm environments and retail products in Oklahoma. Journal of Food Protection, 68(10), 2022–2029.
Wu, H., Liu, B., Liu, J., Pan, Y., Yuan, L., & Hu, G. (2012). Phenotypic and molecular characterization of CTX-M-14 extended-spectrum beta-lactamase and plasmid-mediated ACT-like AmpC beta-lactamase produced by Klebsiella pneumoniae isolates from chickens in Henan Province, China. Genetics Molecular Research, 11(3), 3357–3364.
Winokur, P., Canton, R., Casellas, J.-M., & Legakis, N. (2001). Variations in the prevalence of strains expressing an extended-spectrum β-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clinical Infectious Diseases, 32(Supplement_2), S94–S103.
Abdallah, N. M. A., Elsayed, S. B., Mostafa, M. M. Y., & El-gohary, G. M. (2011). Biofilm forming bacteria isolated from urinary tract infection, relation to catheterization and susceptibility to antibiotics. International Journal of Biotechnology Molecular Biology Research, 2(10), 172–178.
Savas, L., Guvel, S., Onlen, Y., Savas, N., & Duran, N. (2006). Nosocomial urinary tract infections: micro-organisms, antibiotic sensitivities and risk factors. West Indian Medical Journal, 55(3), 188.
Poonguzhali, S., Madhaiyan, M., & Sa, T. (2007). Production of acyl-homoserine lactone quorum-sensing signals is wide-spread in gram-negative Methylobacterium. Journal of Microbiology Biotechnology, 17(2), 226–233.
Wang, L.-H., Weng, L.-X., Dong, Y.-H., & Zhang, L.-H. (2004). Specificity and enzyme kinetics of the quorum-quenching N-acyl homoserine lactone lactonase (AHL-lactonase). Journal of Biological Chemistry, 279(14), 13645–13651.
Mohammed Sakr, M., Mohamed Anwar Aboshanab, K., Mabrouk Aboulwafa, M., & Abdel-Haleem Hassouna, N. (2013). Characterization and complete sequence of lactonase enzyme from Bacillus weihenstephanensis isolate P65 with potential activity against acyl homoserine lactone signal molecules. BioMed Research International, 2013, 1–10.
Alshammari, T. M., Al-Hassan, A. A., Hadda, T. B., & Aljofan, M. (2015). Comparison of different serum sample extraction methods and their suitability for mass spectrometry analysis. Saudi Pharmaceutical Journal, 23(6), 689–697.
See-Too, W. S., Convey, P., Pearce, D. A., & Chan, K.-G. (2018). Characterization of a novel N-acylhomoserine lactonase, AidP, from Antarctic Planococcus sp. Microbial Cell Factories, 17(1), 179.
Vega, L. M., Mathieu, J., Yang, Y., Pyle, B. H., McLean, R. J., & Alvarez, P. J. (2014). Nickel and cadmium ions inhibit quorum sensing and biofilm formation without affecting viability in Burkholderia multivorans. International Biodeterioration Biodegradation, 91, 82–87.
Ortori, C. A., Dubern, J.-F., Chhabra, S. R., Cámara, M., Hardie, K., Williams, P., & Barrett, D. A. (2011). Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4 (1H)-quinolone families of quorum-sensing signaling molecules using LC-MS/MS. Analytical Bioanalytical Chemistry, 399(2), 839–850.
Yates, E. A., Philipp, B., Buckley, C., Atkinson, S., Chhabra, S. R., Sockett, R. E., Goldner, M., Dessaux, Y., Cámara, M., Smith, H., & Williams, P. (2002). N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infection Immunity, 70(10), 5635–5646.
Leipert, J., Treitz, C., Leippe, M., & Tholey, A. (2017). Identification and quantification of N-acyl homoserine lactones involved in bacterial communication by small-scale synthesis of internal standards and matrix-assisted laser desorption/ionization mass spectrometry. Journal of The American Society for Mass Spectrometry, 28(12), 2538–2547.
Wang, J., Quan, C., Wang, X., Zhao, P., & Fan, S. (2011). Extraction, purification and identification of bacterial signal molecules based on N-acyl homoserine lactones. Microbial Biotechnology, 4(4), 479–490.
Fekete, A., Frommberger, M., Rothballer, M., Li, X., Englmann, M., Fekete, J., Hartmann, A., Eberl, L., & Schmitt-Kopplin, P. (2007). Identification of bacterial N-acylhomoserine lactones (AHLs) with a combination of ultra-performance liquid chromatography (UPLC), ultra-high-resolution mass spectrometry, and in-situ biosensors. Analytical Bioanalytical Chemistry, 387(2), 455–467.
Cruz, E., Euerby, M., Johnson, C., & Hackett, C. (1997). Chromatographic classification of commercially available reverse-phase HPLC columns. Chromatographia, 44(3-4), 151–161.
Wang, H., Cai, T., Weng, M., Zhou, J., Cao, H., Zhong, Z., & Zhu, J. (2006). Conditional production of acyl-homoserine lactone-type quorum-sensing signals in clinical isolates of enterobacteria. Journal of Medical Microbiology, 55(12), 1751–1753.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Reham A. Hosny designed the workflow, performed cultural isolation of K. pneumoniae and biosensor detection of acyl-homoserine lactone molecules, and performed all statistical analyses throughout the whole manuscript. Mai A. Fadel developed an RP-HPLC method for the detection of N-acyl-homoserine lactone molecules and statistically analyzed validated method results. Reham A. Hosny and Mai A. Fadel wrote, revised, and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hosny, R.A., Fadel, M.A. Detection of Quorum Sensing N-Acyl-Homoserine Lactone Molecules Produced by Different Resistant Klebsiella pneumoniae Isolates Recovered from Poultry and Different Environmental Niches. Appl Biochem Biotechnol 193, 3351–3370 (2021). https://doi.org/10.1007/s12010-021-03605-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03605-w