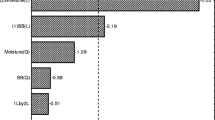
Abstract
Soluble coffee, being one of the world’s most popular consuming drinks, produces a considerable amount of spent coffee ground (SCG) along with its production. The SCG could function as a potential lignocellulosic feedstock for production of bioproducts. The objective of this study is to investigate the possible optimal condition of dilute acid hydrolysis (DAH) at high solids and mild temperature condition to release the reducing sugars from SCG. The optimal condition was found to be 5.3 % (w/w) sulfuric acid concentration and 118 min reaction time. Under the optimal condition, the mean yield of reducing sugars from enzymatic saccharification of defatted SCG acid hydrolysate was 563 mg/g. The SCG hydrolysate was then successfully applied to culture Lipomyces starkeyi for microbial oil fermentation without showing any inhibition. The results suggested that dilute acid hydrolysis followed by enzymatic saccharification has the great potential to convert SCG carbohydrates to reducing sugars. This study is useful for the further develo** of biorefinery using SCG as feedstock at a large scale.



Similar content being viewed by others
References
Singh, V., Mani, I., Chaudhary, D.K., et al. (2013). Metabolic engineering of biosynthetic pathway for production of renewable biofuels. Applied Biochemistry and Biotechnology, 172(3), 1158–1171.
Porter, J.R., **e, L., Challinor, A.J., et al. (2014). Food security and food production systems. In C. B. Field et al. (Eds.), Climate change 2014: impacts, adaptation, and vulnerability. Part a: global and sectoral aspects. Contribution of working group II to the fifth assessment report of the intergovernmental panel of climate change (pp. 485–533). Cambridge: Cambridge University Press.
Ghosh, D., Dasgupta, D., Agrawal, D., et al. (2015). Fuels and chemicals from lignocellulosic biomass: an integrated biorefinery approach. Energy & Fuels, 29(5), 3149–3157.
Xu, Z., & Huang, F. (2014). Pretreatment methods for bioethanol production. Applied Biochemistry and Biotechnology, 174(1), 43–62.
Pleissner, D., Qi, Q., Gao, C., et al. (2016). Valorization of organic residues for the production of added value chemicals: a contribution to the bio-based economy. Biochemical Engineering Journal. doi:10.1016/j.bej.2015.12.016.
Elliston, A., Collins, S.R.A., Faulds, C.B., et al. (2014). Biorefining of waste paper biomass: increasing the concentration of glucose by optimising enzymatic hydrolysis. Applied Biochemistry and Biotechnology, 172(7), 3621–3634.
Sun, Z., Li, M., Qi, Q., et al. (2014). Mixed food waste as renewable feedstock in succinic acid fermentation. Applied Biochemistry and Biotechnology, 174(5), 1822–1833.
Cheng, Y.-S., Chen, K.-Y., & Chou, T.-H. (2015). Concurrent calcium peroxide pretreatment and wet storage of water hyacinth for fermentable sugar production. Bioresource Technology, 176, 267–272.
Chen, K.-Y., Zheng, Y., & Cheng, Y.-S. (2015). Integrated alkali pretreatment and preservation of wet lettuce (Pistia stratiotes) by lactic acid bacteria for fermentable sugar production. Biomass and Bioenergy, 81, 249–255.
Kajaste, R. (2014). Chemicals from biomass—managing greenhouse gas emissions in biorefinery production chains—a review. Journal of Cleaner Production, 75, 1–10.
Kondamudi, N., Mohapatra, S. K., & Misra, M. (2008). Spent coffee grounds as a versatile source of green energy. Journal of Agricultural and Food Chemistry, 56(24), 11757–11760.
Chiyanzu, I., Brienzo, M., García-Aparicio, M.P., et al. (2014). Application of endo-β-1,4,d-mannanase and cellulase for the release of mannooligosaccharides from steam-pretreated spent coffee ground. Applied Biochemistry and Biotechnology, 172(7), 3538–3557.
Bolton, D., & Global Solubles (2015). STiR tea & coffee. Bangkok: Inter Co., Ltd..
Mussatto, S.I., Machado, E.M.S., Martins, S., et al. (2011). Production, composition, and application of coffee and its industrial residues. Food and Bioprocess Technology, 4(5), 661–672.
Wolfrom, M., Plunkett, R., & Laver, M. (1960). Coffee constituents, carbohydrates of the coffee bean. Journal of Agricultural and Food Chemistry, 8(1), 58–65.
Petrik, S., Obruča, S., Benešová, P., et al. (2014). Bioconversion of spent coffee grounds into carotenoids and other valuable metabolites by selected red yeast strains. Biochemical Engineering Journal, 90, 307–315.
Kwon, E. E., Yi, H., & Jeon, Y. J. (2013). Sequential co-production of biodiesel and bioethanol with spent coffee grounds. Bioresource Technology, 136, 475–480.
Ballesteros, L., Teixeira, J., & Mussatto, S. (2014). Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food and Bioprocess Technology, 7(12), 3493–3503.
Fonseca, D.A., Lupitskyy, R., Timmons, D., et al. (2014). Towards integrated biorefinery from dried distillers grains: selective extraction of pentoses using dilute acid hydrolysis. Biomass and Bioenergy, 71, 178–186.
Gao, X., Kumar, R., Singh, S., et al. (2014). Comparison of enzymatic reactivity of corn stover solids prepared by dilute acid, AFEX™, and ionic liquid pretreatments. Biotechnology for Biofuels, 7, 71.
Chandel, A.K., Antunes, F.A., de Arruda, P.V., et al. (2012). Dilute acid hydrolysis of agro-residues for the depolymerization of hemicellulose: state-of-the-art. In D-Xylitol. (p. 39–61). Heidelberg: Springer Berlin. doi:10.1007/978-3-642-31887-0_2.
Lenihan, P., Orozco, A., O’Neill, E., et al. (2010). Dilute acid hydrolysis of lignocellulosic biomass. Chemical Engineering Journal, 156(2), 395–403.
Chimentão, R.J., Lorente, E., Gispert-Guirado, F., et al. (2014). Hydrolysis of dilute acid-pretreated cellulose under mild hydrothermal conditions. Carbohydrate Polymers, 111, 116–124.
Modenbach, A. A., & Nokes, S. E. (2012). The use of high-solids loadings in biomass pretreatment—a review. Biotechnology and Bioengineering, 109(6), 1430–1442.
Cheng, Y.-S., Zheng, Y., Yu, C., et al. (2010). Evaluation of high solids alkaline pretreatment of rice straw. Applied Biochemistry and Biotechnology, 162(6), 1768–1784.
Xu, J., Du, W., Zhao, X., et al. (2013). Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuels, Bioproducts and Biorefining, 7(1), 65–77.
Huang, C., Chen, X.-f., **ong, L., et al. (2013). Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnology Advances, 31(2), 129–139.
Zheng, Y., Yu, X., Zeng, J., et al. (2012). Feasibility of filamentous fungi for biofuel production using hydrolysate from dilute sulfuric acid pretreatment of wheat straw. Biotechnology for Biofuels, 5(1), 50.
Gong, Z., Wang, Q., Shen, H., et al. (2012). Co-fermentation of cellobiose and xylose by Lipomyces starkeyi for lipid production. Bioresource Technology, 117, 20–24.
Wood, I.P., Elliston, A., Ryden, P., et al. (2012). Rapid quantification of reducing sugars in biomass hydrolysates: improving the speed and precision of the dinitrosalicylic acid assay. Biomass and Bioenergy, 44, 117–121.
Cheng, Y.-S., Zheng, Y., & VanderGheynst, J. S. (2010). Rapid quantitative analysis of lipids using a colorimetric method in a microplate format. Lipids, 46(1), 95–103.
Obruca, S., Petrik, S., Benesova, P., et al. (2014). Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Applied Microbiology and Biotechnology, 98(13), 5883–5890.
Yu, J., Porter, M., & Jaremko, M. (2013). Generation and utilization of microbial biomass hydrolysates in recovery and production of poly (3-hydroxybutyrate). Biomass now: cultivation and utilization (p. 33–48). Rijeka: InTech.
Ahring, B.K., Biswas, R., Ahamed, A., et al. (2015). Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresource Technology, 175, 182–188.
Montgomery, L. F., & Bochmann, G. (2014). Pretreatment of feedstock for enhanced biogas production. Ireland: IEA Bioenergy.
Mussatto, S.I., Carneiro, L.M., Silva, J.P.A., et al. (2011). A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydrate Polymers, 83(2), 368–374.
Haghighi Mood, S., Hossein Golfeshan, A., Tabatabaei, M., et al. (2013). Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renewable and Sustainable Energy Reviews, 27, 77–93.
Jia, J., Yu, B., Wu, L., et al. (2014). Biomass enzymatic saccharification is determined by the non-KOH-extractable wall polymer features that predominately affect cellulose crystallinity in corn. PloS one ,9(9), e108449.
Banerji, A., Balakrishnan, M., & Kishore, V. V. N. (2013). Low severity dilute-acid hydrolysis of sweet sorghum bagasse. Applied Energy, 104, 197–206.
Jooste, T., García-Aparicio, M.P., Brienzo, M., et al. (2013). Enzymatic hydrolysis of spent coffee ground. Applied Biochemistry and Biotechnology, 169(8), 2248–2262.
Choi, I.S., Wi, S.G., Kim, S.-B., et al. (2012). Conversion of coffee residue waste into bioethanol with using pop** pretreatment. Bioresource Technology, 125, 132–137.
Chiyanzy, I., Brienzo, M., García-Aparicio, M., et al. (2015). Spent coffee ground mass solubilisation by steam explosion and enzymatic hydrolysis. Journal of Chemical Technology & Biotechnology, 90(3), 449–458.
Yu, X., Zheng, Y., Dorgan, K.M., et al. (2011). Oil production by oleaginous yeasts using the hydrolysate from pretreatment of wheat straw with dilute sulfuric acid. Bioresource Technology, 102(10), 6134–6140.
Huang, C., Chen, X.-F., Yang, X.-Y., et al. (2013). Bioconversion of corncob acid hydrolysate into microbial oil by the oleaginous yeast Lipomyces starkeyi. Applied Biochemistry and Biotechnology, 172(4), 2197–2204.
Matsakas, L., Sterioti, A.-A., Rova, U., et al. (2014). Use of dried sweet sorghum for the efficient production of lipids by the yeast Lipomyces starkeyi CBS 1807. Industrial Crops and Products, 62, 367–372.
Acknowledgments
This work was supported by grants from the National Science Council and Ministry of Science and Technology, Taiwan (NSC102-2622-E-037-002, and MOST 103-2221-E-224-085).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Figure S1
SEM images of untreated SCG (a), diluted acid treated SCG (b) and diluted acid treated followed by enzymatic treated SCG (c) (DOCX 1.31 mb)
Rights and permissions
About this article
Cite this article
Wang, HM.D., Cheng, YS., Huang, CH. et al. Optimization of High Solids Dilute Acid Hydrolysis of Spent Coffee Ground at Mild Temperature for Enzymatic Saccharification and Microbial Oil Fermentation. Appl Biochem Biotechnol 180, 753–765 (2016). https://doi.org/10.1007/s12010-016-2130-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2130-8