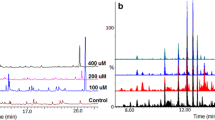
Abstract
Exogenous application of synthetic and natural elicitors of plant defence has been shown to result in mass production of secondary metabolites with nutraceuticals properties in cultured cells. In particular, salicylic acid (SA) treatment has been reported to induce the production of phenylpropanoids, including cinnamic acid derivatives bound to quinic acid (chlorogenic acids). Centella asiatica is an important medicinal plant with several therapeutic properties owing to its wide spectrum of secondary metabolites. We investigated the effect of SA on C. asiatica cells by monitoring perturbation of chlorogenic acids in particular. Different concentrations of SA were used to treat C. asiatica cells, and extracts from both treated and untreated cells were analysed using an optimised UHPLC-QTOF-MS/MS method. Semi-targeted multivariate data analyses with the aid of principal component analysis (PCA) and orthogonal projection to latent structures-discriminant analysis (OPLS-DA) revealed a concentration-dependent metabolic response. Surprisingly, a range of chlorogenic acid derivatives were found to be downregulated as a consequence of SA treatment. Moreover, irbic acid (3,5-O-dicaffeoyl-4-O-malonilquinic acid) was found to be a dominant CGA in C. asiatica cells, although the SA treatment also had a negative effect on its concentration. Overall SA treatment was found to be an ineffective elicitor of CGA production in cultured C. asiatica cells.




Similar content being viewed by others
References
Verpoorte, R., Contin, A., & Memelink, J. (2002). Biotechnology for the production of plant secondary metabolites. Phytochemistry Reviews, 1, 13–25. doi:10.1023/A:1015871916833.
Kolewe, M. E., Gaurav, V., & Roberts, S. C. (2008). Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Molecular Pharmaceutics, 5, 243–256. doi:10.1021/mp7001494.
Gallego, A., Ramirez-Estrada, K., Vidal-Limon, H. R., Hidalgo, D., Lalaleo, L., Khan Kayani, W., Cusido, R. M., & Palazon, J. (2014). Biotechnological production of centellosides in cell cultures of Centella asiatica (L) Urban. Engineering in Life Sciences, 14, 633–642. doi:10.1002/elsc.201300164.
Gupta, A., Verma, S., Kushwaha, P., Srivastava, S., & Aks, R. (2014). Quantitative estimation of asiatic acid, asiaticoside & madecassoside in two accessions of Centella asiatica (L) Urban for morpho-chemotypic variation. Indian Journal of Pharmaceutical Education and Research, 48, 75–79. doi:10.5530/ijper.48.3.9.
Mangas, S., Bonfill, M., Osuna, L., Moyano, E., Tortoriello, J., Cusido, R. M., Teresa Piñol, M., & Palazón, J. (2006). The effect of methyl jasmonate on triterpene and sterol metabolisms of Centella asiatica, Ruscus aculeatus and Galphimia glauca cultured plants. Phytochemistry, 67, 2041–2049. doi:10.1016/j.phytochem.2006.06.025.
Pieterse, C. M. J., Leon-Reyes, A., Van der Ent, S., & Van Wees, S. C. M. (2009). Networking by small-molecule hormones in plant immunity. Nature Chemical Biology, 5, 308–316. doi:10.1038/nchembio.164.
Hayat, Q., Hayat, S., Irfan, M., & Ahmad, A. (2010). Effect of exogenous salicylic acid under changing environment: a review. Environmental and Experimental Botany, 68, 14–25. doi:10.1016/j.envexpbot.2009.08.005.
Ali, M. B., Hahn, E. J., & Paek, K. Y. (2007). Methyl jasmonate and salicylic acid induced oxidative stress and accumulation of phenolics in Panax ginseng bioreactor root suspension cultures. Molecules, 12, 607–621. doi:10.3390/12030607.
Rodas-Junco, B. A., Cab-Guillén, Y., Muñoz-Sánchez, J. A., Vázquez-Flota, F., Monforte-González, M., & Hernández-Sotomayor, S. M. T. (2013). Salicylic acid induces vanillin synthesis through the phospholipid signaling pathway in Capsicum chinense cell cultures. Plant Signaling & Behavior, 8, e26752–1–8. doi:10.4161/psb.26752.
James, J. T., & Dubery, I. A. (2009). Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.) Urban. Molecules, 14, 3922–3941. doi:10.3390/molecules14103922.
Oyedeji, O. A., & Afolayan, A. J. (2005). Chemical composition and antibacterial activity of the essential oil of Centella asiatica growing in South Africa. Pharmaceutical Biology, 43, 249–252. doi:10.1080/13880200590928843.
James, J. T., Meyer, R., & Dubery, I. A. (2008). The occurrence of triterpenoid saponins in callus, cell suspensions and leaves of two Southern African phenotypes of Centella asiatica. Plant Cell, Tissue and Organ Culture, 94, 91–99.
James, J. T., Tugizimana, F., Steenkamp, P. A., & Dubery, I. A. (2013). Metabolomic analysis of methyl jasmonate-induced triterpenoid production in the medicinal herb Centella asiatica (L.) Urban. Molecules, 18, 4267–4281. doi:10.3390/molecules18044267.
Kim, O. T., Um, Y., **, M. L., Kim, Y. C., Bang, K. H., Hyun, D. Y., Lee, H. S., & Lee, Y. (2013). Analysis of expressed sequence tags from Centella asiatica (L.) Urban hairy roots elicited by methyl jasmonate to discover genes related to cytochrome P450s and glucosyltransferases. Plant Biotechnology Reports, 8, 211–220. doi:10.1007/s11816-013-0311-2.
Raoseta, O., Rodier-goud, M., Rivallan, R., Lussert, A., Danthu, P., Lamotte, F. D., Ramavovololona, P., Noyer, J.-L., & Baurens, F. (2013). Insight into the biology, genetics and evolution of the Centella asiatica polyploid complex in Madagascar. Industrial Crops and Products, 47, 118–125. doi:10.1016/j.indcrop.2013.02.022.
Maulidiani, Abas, F., Khatib, A., Shaari, K., & Lajis, N. H. (2014). Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Industrial Crops and Products, 55, 238–247. doi:10.1016/j.indcrop.2014.02.013.
Tugizimana, F., Ncube, E. N., Steenkamp, P. A., & Dubery, I. A. (2015). Metabolomics-derived insights into the manipulation of terpenoid synthesis in Centella asiatica cells by methyl jasmonate. Plant Biotechnology Reports, 9, 125–136. doi:10.1007/s11816-015-0350-y.
Govarthanan, M., Ra**ikanth, R., Kamala-Kannan, S., & Selvankumar, T. (2015). A comparative study on bioactive constituents between wild and in vitro propagated Centella asiatica. Journal of Genetic Engineering and Biotechnology, 13, 25–29. doi:10.1016/j.jgeb.2014.12.003.
Bylka, W., Znajdek-Awiżeń, P., Studzińska-Sroka, E., Dańczak-Pazdrowska, A., & Brzezińska, M. (2014). Centella asiatica in dermatology: an overview. Phytotherapy Research, 28, 7–1124. doi:10.1002/ptr.5110.
Omar, N. S., Akmal, Z., Zakaria, C., & Mian, T. S. (2011). Centella asiatica modulates neuron cell survival by altering caspase-9 pathway. Journal of Medicinal Plants Research, 5, 2201–2209.
Soumyanath, A., Zhong, Y. P., Henson, E., Wadsworth, T., Bishop, J., Gold, B. G., & Quinn, J. F. (2012). Centella asiatica extract improves behavioral deficits in a mouse model of Alzheimer’s disease: investigation of a possible mechanism of action. International Journal of Alzheimers Disease, 2012, 381974. doi:10.1155/2012/381974.
Gray, N. E., Morré, J., Kelley, J., Maier, C. S., Stevens, J. F., Joseph, F., & Soumyanath, A. (2014). Caffeoylquinic acids in Centella asiatica protect against β-amyloid toxicity. Journal of Alzheimers Disease, 40, 359–373. doi:10.3233/JAD-131913.
Dhanasekaran, M., Holcomb, L., Hitt, A., Tharakhan, B., Porter, J., Young, K., & Manyam, B. (2009). Centella asiatica extract selectively decreases amyloid beta levels in hippocampus of Alzheimer’s disease animal model. Phytotherapy Research, 23, 14–19. doi:10.1002/ptr.
Santos, R. M. M., Hunter, T., Wright, N., & Andrade, L. D. R. (2013). Caffeine and chlorogenic acids in coffee and effects on selective neurodegenerative diseases. Journal of Pharmaceutical and Scientific Innovation, 2, 9–17.
Clifford, M. N. (2000). Review chlorogenic acids and other cinnamates—nature, occurrence, dietary burden, absorption and metabolism. Journal of the Science of Food and Agriculture, 80, 1033–1043. doi:10.1002/(SICI)1097-0010(20000515)80:7<1033::AID-JSFA595>3.0.CO;2-T.
Marques, V., & Farah, A. (2009). Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chemistry, 113, 1370–1376. doi:10.1016/j.foodchem.2008.08.086.
Koshiro, Y., Jackson, M. C., Katahira, R., Wang, M. L., Nagai, C., & Ashihara, H. (2007). Biosynthesis of chlorogenic acids in growing and ripening fruits of Coffea arabica and Coffea canephora plants. Zeitschrift für Naturforschung—Section C, 62, 731–742.
Plazas, M., Andújar, I., Vilanova, S., Hurtado, M., Gramazio, P., Herraiz, F. J., & Prohens, J. (2013). Breeding for chlorogenic acid content in eggplant: interest and prospects. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 41, 26–35.
Alakolanga, A. G. A. W., Siriwardene, A. M. D., Savitri Kumar, N., Jayasinghe, L., Jaiswal, R., & Kuhnert, N. (2014). LC-MSn identification and characterization of the phenolic compounds from the fruits of Flacourtia indica (Burm. F.) Merr. and Flacourtia inermis Roxb. Food Research International, 62, 388–396. doi:10.1016/j.foodres.2014.03.036.
Long, H. S., Stander, M. A., & Van Wyk, B. E. (2012). Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. South African Journal of Botany, 82, 53–59. doi:10.1016/j.sajb.2012.07.017.
Antognoni, F., Perellino, N. C., Crippa, S., Dal Toso, R., Danieli, B., Minghetti, A., Poli, F., & Pressi, G. (2011). Irbic acid, a dicaffeoylquinic acid derivative from Centella asiatica cell cultures. Fitoterapia, 82, 950–954. doi:10.1016/j.fitote.2011.05.008.
Mhlongo, M. I., Piater, L. A., Steenkamp, P. A., Madala, N. E., & Dubery, I. A. (2014). Priming agents of plant defence stimulate the accumulation of mono- and di-acylated quinic acids in cultured tobacco cells. Physiological and Molecular Plant Pathology, 88, 61–66. doi:10.1016/j.pmpp.2014.09.002.
Sanabria, N. M., & Dubery, I. A. (2006). Differential display profiling of the Nicotiana response to LPS reveals elements of plant basal resistance. Biochemical and Biophysical Research Communications, 344, 1001–1007. doi:10.1016/j.bbrc.2006.03.216.
Ncube, E. N., Mhlongo, M. I., Piater, L. A., Steenkamp, P. A., Dubery, I. A., & Madala, N. E. (2014). Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chemistry Central Journal, 8, 1–10. doi:10.1186/s13065-014-0066-z.
Clifford, M. N., Knight, S., & Kuhnert, N. (2005). Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. Journal of Agricultural and Food Chemistry, 53, 3821–3832. doi:10.1021/jf050046h.
Clifford, M. N., Kirkpatrick, J., Kuhnert, N., Roozendaal, H., & Salgado, P. R. (2008). LC-MSn analysis of the cis isomers of chlorogenic acids. Food Chemistry, 106, 379–385. doi:10.1016/j.foodchem.2007.05.081.
Jaiswal, R., Müller, H., Müller, A., Karar, M. G. E., & Kuhnert, N. (2014). Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC–MSn. Phytochemistry, 108, 252–263. doi:10.1016/j.phytochem.2014.08.023.
Sumner, L. W., Amberg, A., Barrett, D., Beale, M. H., Beger, R., Daykin, C. A., et al. (2007). Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG), Metabolomics Standards Initiative (MSI). Metabolomics, 3, 211–221. doi:10.1007/s11306-007-0082-2.
Trygg, J., Holmes, E., & Lundstedt, T. (2007). Chemometrics in metabonomics. Journal of Proteome Research, 6, 469–479. doi:10.1021/pr060594q.
Wiklund, S., Johansson, E., Sjöström, L., Mellerowicz, E. J., Edlund, U., Shockcor, J. P., Gottfries, J., Moritz, T., & Trygg, J. (2008). Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Analytical Chemistry, 80, 115–122. doi:10.1021/ac0713510.
Galindo-Prieto, B., Eriksson, L., & Trygg, J. (2014). Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). Journal of Chemometrics, 28, 623–632. doi:10.1002/cem.2627.
Madala, N. E., Steenkamp, P. A., Piater, L. A., & Dubery, I. A. (2014). Metabolomic insights into the bioconversion of isonitrosoacetophenone in Arabidopsis thaliana and its effects on defense-related pathways. Plant Physiology and Biochemistry, 84, 87–95. doi:10.1016/j.plaphy.2014.08.023.
Saccenti, E., Hoefsloot, H. C. J., Smilde, A. K., Westerhuis, J. A., & Hendriks, M. M. W. B. (2014). Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics, 10, 361–374. doi:10.1007/s11306-013-0598-6.
Zhang, J. Y., Zhang, Q., Li, N., Wang, Z. J., Lu, J. Q., & Qiao, Y. J. (2013). Diagnostic fragment-ion-based and extension strategy coupled to DFIs intensity analysis for identification of chlorogenic acids isomers in Flos Lonicerae Japonicae by HPLC-ESI-MSn. Talanta, 104, 1–9. doi:10.1016/j.talanta.2012.11.012.
Shukri, M. M., Alan, C., & Noorzuraini, R. S. (2011). Polyphenols and antioxidant activities of selected traditional vegetables. Journal of Tropical Agriculture and Food Science, 39, 69–83.
Alonso-Salces, R. M., Guillou, C., & Berrueta, L. A. (2009). Liquid chromatography coupled with ultraviolet absorbance detection, electrospray ionization, collision-induced dissociation and tandem mass spectrometry on a triple quadrupole for the on-line characterization of polyphenols and methylxanthines in green coffee beans. Rapid Communications in Mass Spectrometry, 23, 363–383. doi:10.1002/rcm.3884.
Jaiswal, R., Deshpande, S., & Kuhnert, N. (2011). Profiling the chlorogenic acids of Rudbeckia hirta, Helianthus tuberosus, Carlina acaulis and Symphyotrichum novae-angliae leaves by LC-MSn. Phytochemical Analysis, 22, 432–441. doi:10.1002/pca.1299.
Satake, T., Kamiya, K., An, Y., Oishi Nee Taka, T., & Yamamoto, J. (2007). The anti-thrombotic active constituents from Centella asiatica. Biological and Pharmaceutical Bulletin, 30, 935–40. doi:10.1248/bpb.30.935.
Plazonić, A., Bucar, F., Maleŝ, Z., Mornar, A., Nigović, B., & Kujundẑić, N. (2009). Identification and quantification of flavonoids and phenolic acids in burr parsley (Caucalis platycarpos L.), using high-performance liquid chromatography with diode array detection and electrospray ionization mass spectrometry. Molecules, 14, 2466–2490. doi:10.3390/molecules14072466.
Nandutu, A. M., Clifford, M., & Howell, N. Z. (2007). Analysis of phenolic compounds in Ugandan sweet potato varieties (NSP, SPK and TZ). African Journal of Biochemistry Research, 1, 29–36.
Jaiswal, R., Patras, M. A., Eravuchira, P. J., & Kuhnert, N. (2010). Profile and characterization of the chlorogenic acids in green Robusta coffee beans by LC-MSn: identification of seven new classes of compounds. Journal of Agricultural and Food Chemistry, 58, 8722–8737. doi:10.1021/jf1014457.
Acknowledgments
The authors would like to thank the South African National Research Foundation and the University of Johannesburg for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Figure S1
Multivariate data analysis of the ESI negative data, showing the orthogonal projection to latent structure-discriminant analysis (OPLS-DA) generated from SA-treated C. asiatica cells incubated for 24 h. Scores plot (A) and S-plot (B) comparing the non-treated cells vs. the 100-μM SA treatment and the scores plot (C) and S-plot (D) comparing the non-treated cells vs. the 200-μM SA treatment. The mass ions in the upper right quadrant of the S-plot are positively correlated to the SA treatment whilst the ions in the lower quadrant are negatively related to the treatment. (GIF 517 kb)
Figure S2
UHPLC-QTOF-MS fragmentation spectra. Showing (A) trans-5-feruloylquinic acid, (B) 3,5 di-caffeoylquinic acid, (C) irbic acid (3,5-O-dicaffeoyl-4-O-malonilquinic acid) and (D) 3-caffeoyl, 5-feruloylquinic acid. (GIF 47 kb)
Rights and permissions
About this article
Cite this article
Ncube, E.N., Steenkamp, P.A., Madala, N.E. et al. Chlorogenic Acids Biosynthesis in Centella asiatica Cells Is not Stimulated by Salicylic Acid Manipulation. Appl Biochem Biotechnol 179, 685–696 (2016). https://doi.org/10.1007/s12010-016-2024-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2024-9