Abstract
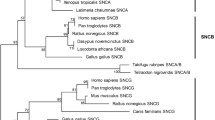
The synaptic protein alpha-synuclein (α-syn) is associated with a number of neurodegenerative diseases, and homology analyses among many species have been reported. Nevertheless, little is known about the cDNA sequence and protein structure of α-syn in tree shrews, and this information might contribute to our understanding of its role in both health and disease. We designed primers to the human α-syn cDNA sequence; then, tree shrew α-syn cDNA was obtained by RT-PCR and sequenced. Based on the acquired tree shrew α-syn cDNA sequence, both the amino acid sequence and the spatial structure of α-syn were predicted and analyzed. The homology analysis results showed that the tree shrew cDNA sequence matches the human cDNA sequence exactly except at nucleotide positions 45, 60, 65, 69, 93, 114, 147, 150, 157, 204, 252, 270, 284, 298, 308, and 324. Further protein sequence analysis revealed that the tree shrew α-syn protein sequence is 97.1 % identical to that of human α-syn. The secondary protein structure of tree shrew α-syn based on random coils and α-helices is the same as that of the human structure. The phosphorylation sites are highly conserved, except the site at position 103 of tree shrewα-syn. The predicted spatial structure of tree shrew α-syn is identical to that of human α-syn. Thus, α-syn might have a similar function in tree shrew and in human, and tree shrew might be a potential animal model for studying the pathogenesis of α-synucleinopathies.




Similar content being viewed by others
References
Arnold, K., Bordoli, L., Kopp, J., & Schwede, T. (2006). The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics, 22, 195–201.
Auluck, P. K., Caraveo, G., & Lindquist, S. (2010). Alpha-synuclein: membrane interactions and toxicity in Parkinson’s disease. Annual Review of Cell and Developmental Biology, 26, 211–233.
Bartolomucci, A., de Biurrun, G., & Fuchs, E. (2001). How tree shrews (Tupaia belangeri) perform in a searching task: evidence for strategy use. Journal of Comparative Psychology, 115, 344–350.
Blom, N., Gammeltoft, S., & Brunak, S. (1999). Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. Journal of Molecular Biology, 294, 1351–1362.
Bussell Jr., R., & Eliezer, D. (2001). Residual structure and dynamics in Parkinson’s disease-associated mutants of alpha-synuclein. The Journal of Biological Chemistry, 276, 45996–46003.
Cao, J., Yang, E. B., Su, J. J., Li, Y., & Chow, P. (2003). The tree shrews: adjuncts and alternatives to primates as models for biomedical research. Journal of Medical Primatology, 32, 123–130.
Conway, K. A., Harper, J. D., & Lansbury Jr., P. T. (2000). Fibrils formed in vitro from alpha-synuclein and two mutant forms linked to Parkinson’s disease are typical amyloid. Biochemistry, 39, 2552–2563.
Cooper, A. A., Gitler, A. D., Cashikar, A., Haynes, C. M., Hill, K. J., Bhullar, B., Liu, K., Xu, K., Strathearn, K. E., Liu, F., Cao, S., Caldwell, K. A., Caldwell, G. A., Marsischky, G., Kolodner, R. D., Labaer, J., Rochet, J. C., Bonini, N. M., & Lindquist, S. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science, 313, 324–328.
Czeh, B., Michaelis, T., Watanabe, T., Frahm, J., de Biurrun, G., van Kampen, M., Bartolomucci, A., & Fuchs, E. (2001). Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proceedings of the National Academy of Sciences of the United States of America, 98, 12796–12801.
Davidson, W. S., Jonas, A., Clayton, D. F., & George, J. M. (1998). Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. The Journal of Biological Chemistry, 273, 9443–9449.
Dickson, D. W., Crystal, H. A., Mattiace, L. A., Masur, D. M., Blau, A. D., Davies, P., Yen, S. H., & Aronson, M. K. (1992). Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiology of Aging, 13, 179–189.
Fuchs, E. (2005). Social stress in tree shrews as an animal model of depression: an example of a behavioral model of a CNS disorder. CNS Spectrums, 10, 182–190.
Fuchs, E., & Flugge, G. (2002). Social stress in tree shrews: effects on physiology, brain function, and behavior of subordinate individuals. Pharmacology, Biochemistry, and Behavior, 73, 247–258.
Fuchs, E., Uno, H., & Flugge, G. (1995). Chronic psychosocial stress induces morphological alterations in hippocampal pyramidal neurons of the tree shrew. Brain Research, 673, 275–282.
Giasson, B. I., Murray, I. V., Trojanowski, J. Q., & Lee, V. M. (2001). A hydrophobic stretch of 12 amino acid residues in the middle of alpha-synuclein is essential for filament assembly. The Journal of Biological Chemistry, 276, 2380–2386.
Gould, E., McEwen, B. S., Tanapat, P., Galea, L. A., & Fuchs, E. (1997). Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. The Journal of Neuroscience, 17, 2492–2498.
Guex, N., & Peitsch, M. C. (1997). SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis, 18, 2714–2723.
Hong, L., Ko, H. W., Gwag, B. J., Joe, E., Lee, S., Kim, Y. T., & Suh, Y. H. (1998). The cDNA cloning and ontogeny of mouse alpha-synuclein. Neuroreport, 9, 1239–1243.
Jakes, R., Spillantini, M. G., & Goedert, M. (1994). Identification of two distinct synucleins from human brain. FEBS Letters, 345, 27–32.
Kruger, R., Kuhn, W., Muller, T., Woitalla, D., Graeber, M., Kosel, S., Przuntek, H., Epplen, J. T., Schols, L., & Riess, O. (1998). Ala30Pro mutation in the gene encoding alpha-synuclein in Parkinson’s disease. Nature Genetics, 18, 106–108.
Lavedan, C. (1998). The synuclein family. Genome Research, 8, 871–880.
Lee, S. B., Park, S. M., Ahn, K. J., Chung, K. C., Paik, S. R., & Kim, J. (2009). Identification of the amino acid sequence motif of alpha-synuclein responsible for macrophage activation. Biochemical and Biophysical Research Communications, 381, 39–43.
Li, J., Uversky, V. N., & Fink, A. L. (2001). Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human alpha-synuclein. Biochemistry, 40, 11604–11613.
Maroteaux, L., Campanelli, J. T., & Scheller, R. H. (1988). Synuclein: a neuron-specific protein localized to the nucleus and presynaptic nerve terminal. The Journal of Neuroscience, 8, 2804–2815.
McCoy, P., Norton, T. T., & McMahon, L. L. (2008). Layer 2/3 synapses in monocular and binocular regions of tree shrew visual cortex express mAChR-dependent long-term depression and long-term potentiation. Journal of Neurophysiology, 100, 336–345.
Mori, F., Nishie, M., Kakita, A., Yoshimoto, M., Takahashi, H., & Wakabayashi, K. (2006). Relationship among alpha-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. Journal of Neuropathology and Experimental Neurology, 65, 808–815.
Norris, E. H., Giasson, B. I., & Lee, V. M. (2004). Alpha-synuclein: normal function and role in neurodegenerative diseases. Current Topics in Developmental Biology, 60, 17–54.
Polymeropoulos, M. H., Lavedan, C., Leroy, E., Ide, S. E., Dehejia, A., Dutra, A., Pike, B., Root, H., Rubenstein, J., Boyer, R., Stenroos, E. S., Chandrasekharappa, S., Athanassiadou, A., Papapetropoulos, T., Johnson, W. G., Lazzarini, A. M., Duvoisin, R. C., Di Iorio, G., Golbe, L. I., & Nussbaum, R. L. (1997). Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science, 276, 2045–2047.
Rospigliosi, C. C., McClendon, S., Schmid, A. W., Ramlall, T. F., Barre, P., Lashuel, H. A., & Eliezer, D. (2009). E46K Parkinson’s-linked mutation enhances C-terminal-to-N-terminal contacts in alpha-synuclein. Journal of Molecular Biology, 388, 1022–1032.
Schwede, T., Kopp, J., Guex, N., & Peitsch, M. C. (2003). SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Research, 31, 3381–3385.
Spillantini, M. G., Schmidt, M. L., Lee, V. M., Trojanowski, J. Q., Jakes, R., & Goedert, M. (1997). Alpha-synuclein in Lewy bodies. Nature, 388, 839–840.
Surguchov, A., McMahan, B., Masliah, E., & Surgucheva, I. (2001). Synucleins in ocular tissues. Journal of Neuroscience Research, 65, 68–77.
Touchman, J. W., Dehejia, A., Chiba-Falek, O., Cabin, D. E., Schwartz, J. R., Orrison, B. M., Polymeropoulos, M. H., & Nussbaum, R. L. (2001). Human and mouse alpha-synuclein genes: comparative genomic sequence analysis and identification of a novel gene regulatory element. Genome Research, 11, 78–86.
Ueda, K., Fukushima, H., Masliah, E., **a, Y., Iwai, A., Yoshimoto, M., Otero, D. A., Kondo, J., Ihara, Y., & Saitoh, T. (1993). Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 90, 11282–11286.
Vekrellis, K., Rideout, H. J., & Stefanis, L. (2004). Neurobiology of alpha-synuclein. Molecular Neurobiology, 30, 1–21.
Wang, J., Zhou, Q., Tian, M., Yang, Y., & Xu, L. (2011). Tree shrew models: a chronic social defeat model of depression and a one-trial captive conditioning model of learning and memory. Zool Res, 32, 24–30.
Xu, L., Fan, Y., Jiang, X., & Yao, Y. (2013). Molecular evidence on the phylogenetic position of tree shrew (Tupia belangeri). Zool Res, 34, 70–76.
Yamashita, A., Fuchs, E., Taira, M., & Hayashi, M. (2010). Amyloid beta (Abeta) protein- and amyloid precursor protein (APP)-immunoreactive structures in the brains of aged tree shrews. Current Aging Science, 3, 230–238.
Zarranz, J. J., Alegre, J., Gomez-Esteban, J. C., Lezcano, E., Ros, R., Ampuero, I., Vidal, L., Hoenicka, J., Rodriguez, O., Atares, B., Llorens, V., Gomez Tortosa, E., del Ser, T., Munoz, D. G., & de Yebenes, J. G. (2004). The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Annals of Neurology, 55, 164–173.
Acknowledgments
This work was supported by the National Natural Science Foundation of China Grants (No. 81274122, 81301073, U1402221), the Specialized Research Fund for the Doctoral Program of Higher Education of China (No. 20121106120056, 20121106130001), Yunnan Natural Science Foundation (No. 2011FB116, 2013FZ132, 2014FB191), the PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (No. 3332013082, 3332013146, 33320140191), Bei**g Natural Science Foundation (No. 7131013), the Fund for the Institute of Medical Biology (No. IMB2013YB01, 2014IMB01ZD), and the Fund for the Institute of Pathogen Biology, Chinese Academy of Medical Sciences (No. 2015IPB201).
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Zheng-Cun Wu and Zhang-Qiong Huang these authors contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wu, Z.C., Huang, ZQ., Jiang, QF. et al. Human and Tree Shrew Alpha-synuclein: Comparative cDNA Sequence and Protein Structure Analysis. Appl Biochem Biotechnol 177, 957–966 (2015). https://doi.org/10.1007/s12010-015-1789-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1789-6