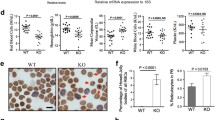
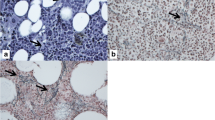
Abstract
Purpose of Review
Recently, there has been an increasing number of studies on the crosstalk between the bone and the bone marrow and how it pertains to anemia. Here, we discuss four heritable clinical syndromes contrasting those in which anemia affects bone growth and development, with those in which abnormal bone development results in anemia, highlighting the multifaceted interactions between skeletal development and hematopoiesis.
Recent Findings
Anemia results from both inherited and acquired disorders caused by either impaired production or premature destruction of red blood cells or blood loss. The downstream effects on bone development and growth in patients with anemia often constitute an important part of their clinical condition. We will discuss the interdependence of abnormal bone development and growth and hematopoietic abnormalities, with a focus on the erythroid lineage. To illustrate those points, we selected four heritable anemias that arise from either defective hematopoiesis impacting the skeletal system (the hemoglobinopathies β-thalassemia and sickle cell disease) versus defective osteogenesis resulting in impaired hematopoiesis (osteopetrosis). Finally, we will discuss recent findings in Diamond Blackfan anemia, an intrinsic disorder of both the erythron and the bone.
Summary
By focusing on four representative hereditary hematopoietic disorders, this complex relationship between bone and blood should lead to new areas of research in the field.






Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Shimamura A, Alter BP. Pathophysiology and management of inherited bone marrow failure syndromes. Blood Rev. 2010;24(3):101–22. https://doi.org/10.1016/j.blre.2010.03.002.
• Vlachos A, Blanc L, Lipton JM. Diamond Blackfan anemia: a model for the translational approach to understanding human disease. Expert Rev Hematol. 2014;7(3):359–72. https://doi.org/10.1586/17474086.2014.897923. (A still timely review describing the pathophysiology of Diamond Blackfan anemia in a clinical context)
Ning S, Zeller MP. Management of iron deficiency. Hematology Am Soc Hematol Educ Program. 2019;2019(1):315–22. https://doi.org/10.1182/hematology.2019000034.
Niss O, Quinn CT. Classification and diagnosis of anemia in children and neonates in Lanzkowsky's Manual of Pediatric Hematology and Oncology, Seventh ed. Fish, JD, Lipton, JM, Lanzkowsky (Eds.), Academic Press, Elsevier Inc. London, United Kingdom.
Toxqui L, Vaquero MP. Chronic iron deficiency as an emerging risk factor for osteoporosis: a hypothesis. Nutrients. 2015;7(4):2324–44. https://doi.org/10.3390/nu7042324.
•• Tsiftsoglou AS. Erythropoietin (EPO) as a key regulator of erythropoiesis, bone remodeling and endothelial transdifferentiation of multipotent mesenchymal stem cells (MSCs): implications in regenerative medicine. Cells. 2021;10(8):2140. https://doi.org/10.3390/cells10082140. (Work describing the seminal observation that erythropoietin drives not only erythropoiesis but also induces osteogenic and endothelial transdifferentiation of mesenchymal stem cell through the erythropoietin receptor signaling pathways, connecting osteogenesis to erythropoiesis)
Kim AR, Ulirsch JC, Wilmes S, Unal E, Moraga I, Karakukcu M, et al. Functional selectivity in cytokine signaling revealed through a pathogenic EPO mutation. Cell. 2017;168(6):1053-64.e15. https://doi.org/10.1016/j.cell.2017.02.026.
Suresh S, Lee J, Noguchi CT. Erythropoietin signaling in osteoblasts is required for normal bone formation and for bone loss during erythropoietin-stimulated erythropoiesis. FASEB J. 2020;34(9):11685–97. https://doi.org/10.1096/fj.202000888R.
Yu VW, Scadden DT. Heterogeneity of the bone marrow niche. Curr Opin Hematol. 2016;23(4):331–8. https://doi.org/10.1097/MOH.0000000000000265.
Kim PG, Niroula A, Shkolnik V, McConkey M, Lin AE, Słabicki M, et al. Dnmt3a-mutated clonal hematopoiesis promotes osteoporosis. J Exp Med. 2021;218(12):e20211872. https://doi.org/10.1084/jem.20211872.
Calvi LM, Link DC. The hematopoietic stem cell niche in homeostasis and disease. Blood. 2015;126(22):2443–51. https://doi.org/10.1182/blood-2015-07-533588.
•• Galán-Díez M, Kousteni S. A bone marrow niche-derived molecular switch between osteogenesis and hematopoiesis. Genes Dev. 2018;32(5–6):324–6. https://doi.org/10.1101/gad.314013.118. (This study describes the mechanism by which specialized bone marrow niche cells regulates the interrelationship between osteogenesis and hematopoiesis)
Aoki K, Kurashige M, Ichii M, Higaki K, Sugiyama T, Kaito T, et al. Identification of CXCL12-abundant reticular cells in human adult bone marrow. Br J Haematol. 2021;193(3):659–68. https://doi.org/10.1111/bjh.17396.
Seike M, Omatsu Y, Watanabe H, Kondoh G, Nagasawa T. Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev. 2018;32(5–6):359–72. https://doi.org/10.1101/gad.311068.117.
Wong P, Fuller PJ, Gillespie MT, Milat F. Bone disease in thalassemia: a molecular and clinical overview. Endocr Rev. 2016;37(4):320–46. https://doi.org/10.1210/er.2015-1105.
• Taher AT, Musallam KM, Cappellini MD. β-thalassemias. N Engl J Med. 2021;384(8):727–43. https://doi.org/10.1056/NEJMra2021838. (This work provides a comprehensive review of the β-thalassemias)
Haidar R, Musallam KM, Taher AT. Bone disease and skeletal complications in patients with β thalassemia major. Bone. 2011;48(3):425–32. https://doi.org/10.1016/j.bone.2010.10.173.
Rund D, Rachmilewitz E. Beta-thalassemia. N Engl J Med. 2005;353(11):1135–46. https://doi.org/10.1056/NEJMra050436.
•• Castro-Mollo M, Gera S, Ruiz-Martinez M, Feola M, Gumerova A, Planoutene M, et al. The hepcidin regulator erythroferrone is a new member of the erythropoiesis-iron-bone circuitry. Elife. 2021;10:e68217. https://doi.org/10.7554/eLife.68217. (This study demonstrated a role for erythroferrone in preventing bone loss during expanded erythropoiesis in beta-thalassemia)
Morabito N, Gaudio A, Lasco A, Atteritano M, Pizzoleo MA, Cincotta M, et al. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: new pieces of the puzzle. J Bone Miner Res. 2004;19(5):722–7. https://doi.org/10.1359/JBMR.040113.
Zaidi M, Kim SM, Mathew M, Korkmaz F, Sultana F, Miyashita S, et al. Bone circuitry and interorgan skeletal crosstalk. Elife. 2023;12:e83142. https://doi.org/10.7554/eLife.83142.
• Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337(11):762–9. https://doi.org/10.1056/NEJM199709113371107. (A timeless and still relevant review of the clinical manifestations of sickle cell disease by a legend in the field)
Mohandas N, Gallagher PG. Red cell membrane: past, present, and future. Blood. 2008;112(10):3939–48. https://doi.org/10.1182/blood-2008-07-161166.
Vanderhave KL, Perkins CA, Scannell B, Brighton BK. Orthopaedic manifestations of sickle cell disease. J Am Acad Orthop Surg. 2018;26(3):94–101. https://doi.org/10.5435/JAAOS-D-16-00255.
DalleCarbonare L, Matte’ A, Valenti MT, Siciliano A, Mori A, Schweiger V, et al. Hypoxia-reperfusion affects osteogenic lineage and promotes sickle cell bone disease. Blood. 2015;126(20):2320–8. https://doi.org/10.1182/blood-2015-04-641969.
Adesina OO, Neumayr LD. Osteonecrosis in sickle cell disease: an update on risk factors, diagnosis, and management. Hematology Am Soc Hematol Educ Program. 2019;2019(1):351–8. https://doi.org/10.1182/hematology.2019000038.
Almeida A, Roberts I. Bone involvement in sickle cell disease. Br J Haematol. 2005;129(4):482–90. https://doi.org/10.1111/j.1365-2141.2005.05476.x.
Sarrai M, Duroseau H, D’Augustine J, Moktan S, Bellevue R. Bone mass density in adults with sickle cell disease. Br J Haematol. 2007;136(4):666–72. https://doi.org/10.1111/j.1365-2141.2006.06487.x.
Eskiocak Ö, Yılmaz M, İlhan G. Metabolic bone diseases in sickle cell anemia patients and evaluation of associated factors. Am J Med Sci. 2022;363(6):490–4. https://doi.org/10.1016/j.amjms.2021.07.002.
Stark Z, Savarirayan R. Osteopetrosis. Orphanet J Rare Dis. 2009;4:5. https://doi.org/10.1186/1750-1172-4-5.
• Wu CC, Econs MJ, DiMeglio LA, Insogna KL, Levine MA, Orchard PJ, et al. Diagnosis and management of osteopetrosis: consensus guidelines from the Osteopetrosis Working Group. J Clin Endocrinol Metab. 2017;102(9):3111–23. https://doi.org/10.1210/jc.2017-01127. (These are important diagnostic and treatment consensus guidelines for osteopetrosis)
Roodman GD. Advances in bone biology: the osteoclast. Endocr Rev. 1996;17(4):308–32. https://doi.org/10.1210/edrv-17-4-308.
Palagano E, Menale C, Sobacchi C, Villa A. Genetics of osteopetrosis. Curr Osteoporos Rep. 2018;16(1):13–25. https://doi.org/10.1007/s11914-018-0415-2.
Sobacchi C, Schulz A, Coxon FP, Villa A, Helfrich MH. Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol. 2013;9(9):522–36. https://doi.org/10.1038/nrendo.2013.137.
Sobacchi C, Abinun M. Osteoclast-poor osteopetrosis. Bone. 2022;164:116541. https://doi.org/10.1016/j.bone.2022.116541.
Del Fattore A, Cappariello A, Teti A. Genetics, pathogenesis and complications of osteopetrosis. Bone. 2008;42(1):19–29. https://doi.org/10.1016/j.bone.2007.08.029.
• Ulirsch JC, Verboon JM, Kazerounian S, Guo MH, Yuan D, Ludwig LS, et al. The genetic landscape of Diamond-Blackfan anemia. Am J Hum Genet. 2018;103(6):930–47. https://doi.org/10.1016/j.ajhg.2018.10.027. (This is an expansive work identifying the genes mutated in Diamond Blackfan anemia)
Narla A, Ebert BL. Ribosomopathies: human disorders of ribosome dysfunction. Blood. 2010;115(16):3196–205. https://doi.org/10.1182/blood-2009-10-178129.
Zhou X, Liao JM, Liao WJ, Lu H. Scission of the p53-MDM2 loop by ribosomal proteins. Genes Cancer. 2012;3(3–4):298–310. https://doi.org/10.1177/1947601912455200.
Dutt S, Narla A, Lin K, Mullally A, Abayasekara N, Megerdichian C, et al. Haploinsufficiency for ribosomal protein genes causes selective activation of p53 in human erythroid progenitor cells. Blood. 2011;117(9):2567–76. https://doi.org/10.1182/blood-2010-07-295238.
Yin Y, Stephen CW, Luciani MG, Fåhraeus R. p53 Stability and activity is regulated by Mdm2-mediated induction of alternative p53 translation products. Nat Cell Biol. 2002;4(6):462–7. https://doi.org/10.1038/ncb801.
Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16(5):369–77. https://doi.org/10.1016/j.ccr.2009.09.024.
Perdahl EB, Naprstek BL, Wallace WC, Lipton JM. Erythroid failure in Diamond-Blackfan anemia is characterized by apoptosis. Blood. 1994;83(3):645–50.
Sakamoto KM, Narla A. Perspective on Diamond-Blackfan anemia: lessons from a rare congenital bone marrow failure syndrome. Leukemia. 2018;32(2):249–51. https://doi.org/10.1038/leu.2017.314.
Vlachos A, Muir E. How I treat Diamond-Blackfan anemia. Blood. 2010;116(19):3715–23. https://doi.org/10.1182/blood-2010-02-251090.
Hom J, Karnavas T, Hartman E, Papoin J, Tang Y, Dulmovits BM, et al. Limb specific failure of proliferation and translation in the mesenchyme leads to skeletal defects in Diamond Blackfan anemia. bioRxiv. 2022;597(7875):256.
Danilova N, Gazda HT. Ribosomopathies: how a common root can cause a tree of pathologies. Dis Model Mech. 2015;8(9):1013–26. https://doi.org/10.1242/dmm.020529.
Gabut M, Bourdelais F, Durand S. Ribosome and translational control in stem cells. Cells. 2020;9(2):497. https://doi.org/10.3390/cells9020497.
Mills EW, Green R. Ribosomopathies: there’s strength in numbers. Science. 2017;358(6363):eaan2755. https://doi.org/10.1126/science.aan2755.
•• Ludwig LS, Gazda HT, Eng JC, Eichhorn SW, Thiru P, Ghazvinian R, et al. Altered translation of GATA1 in Diamond-Blackfan anemia. Nat Med. 2014;20(7):748–53. https://doi.org/10.1038/nm.3557. (This paper demonstrates faulty transcription regulation as a consequence of ribosomal protein haploinsufficency)
Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–54. https://doi.org/10.1016/s0092-8674(00)80257-3.
Acknowledgements
We would like to thank the Saint Baldrick’s Foundation, Pediatric Cancer Foundation, Gambino Medical & Science Foundation, and Diamond Blackfan Anemia Foundation for supporting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
JML reports honoraria/travel support from Bristol Myers Squibb and Chiesi, USA, outside the submitted work. RW, CC, LCW and LB report no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Willimann, R., Chougar, C., Wolfe, L.C. et al. Defects in Bone and Bone Marrow in Inherited Anemias: the Chicken or the Egg. Curr Osteoporos Rep 21, 527–539 (2023). https://doi.org/10.1007/s11914-023-00809-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-023-00809-3