Abstract
Purpose of Review
In this review, we discuss the evidence supporting the effects of statins on mast cells (MCs) in atherosclerosis and their molecular mechanism of action.
Recent Findings
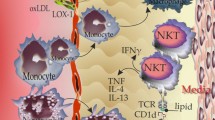
Statins or HMG-CoA reductase inhibitors are known for their lipid-lowering properties and are widely used in the prevention and treatment of cardiovascular diseases. There is growing evidence that statins have an inhibitory effect on MCs, which contributes to the pleiotropic effect of statins in various diseases. MCs are one of the crucial effectors of the immune system which play an essential role in the pathogenesis of multiple disorders. Recent studies have shown that MCs are involved in the development of atherosclerotic plaques. MCs secrete various inflammatory cytokines (IL-6, IL4, TNF-α, and IFNγ) and inflammatory mediators (histamine, tryptase, proteoglycans) after activation by various stimulants. This, in turn, will exacerbate atherosclerosis. Statins suppress the activation of MCs via IgE inhibition which leads to inhibition of inflammatory mediators and cytokines which are involved in the development and progression of atherosclerosis.
Summary
In kee** with this evidence presented here, MCs can be considered as one of the therapeutic targets for statins in the treatment of atherosclerosis.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Wilcock A, Bahri R, Bulfone-Paus S, Arkwright PD. Mast cell disorders: from infancy to maturity. Allergy. 2019;74(1):53–63.
Lagraauw HM (2015) Stress-induced modulation of the innate immune system in cardiovascular disease. Biopharmacy division, Leiden Academic Center for Drug Research (LACDR …,
Kritikou E, Kuiper J, Kovanen PT, Bot I. The impact of mast cells on cardiovascular diseases. Eur J Pharmacol. 2016;778:103–15.
Bachelet I, Levi-Schaffer F, Mekori YA. Mast cells: not only in allergy. Immunol Allergy Clin. 2006;26(3):407–25.
Krauth M, Majlesi Y, Sonneck K, Samorapoompichit P, Ghannadan M, Hauswirth A, et al. Effects of various statins on cytokine-dependent growth and IgE-dependent release of histamine in human mast cells. Allergy. 2006;61(3):281–8.
Meyer N, Zenclussen AC. Mast cells—good guys with a bad image? Am J Reprod Immunol. 2018;80(4):e13002.
Ringvall M, Rönnberg E, Wernersson S, Duelli A, Henningsson F, Åbrink M, et al. Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol. 2008;121(4):1020–6.
Krystel-Whittemore M, Dileepan KN, Wood JG. Mast cell: a multi-functional master cell. Front Immunol. 2016;6:620.
Roy A. Mast cells as sentinels: role of serglycin and mast cell proteases in infection and inflammation: Acta Universitatis Upsaliensis; 2012.
Wernersson S, Pejler G. Mast cell secretory granules: armed for battle. Nat Rev Immunol. 2014;14(7):478–94.
Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2):S73–80.
Moon TC, Befus AD, Kulka M. Mast cell mediators: their differential release and the secretory pathways involved. Front Immunol. 2014;5:569.
Ashmole I, Bradding P. Ion channels regulating mast cell biology. Clin Exp Allergy. 2013;43(5):491–502.
Yeganeh B, Wiechec E, Ande SR, Sharma P, Moghadam AR, Post M, et al. Targeting the mevalonate cascade as a new therapeutic approach in heart disease, cancer and pulmonary disease. Pharmacol Ther. 2014;143(1):87–110.
Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, García FA, et al. Statin use for the primary prevention of cardiovascular disease in adults: US preventive services task force recommendation statement. Jama. 2016;316(19):1997–2007.
Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388(10059):2532–61.
Parizadeh SM, Azarpazhooh MR, Moohebati M, Nematy M, Ghayour-Mobarhan M, Tavallaie S, et al. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. 2011;46(4):333–40.
Yaribeygi H, Faghihi N, Mohammadi MT, Sahebkar A. Effects of atorvastatin on myocardial oxidative and nitrosative stress in diabetic rats. Comp Clin Pathol. 2018;27(3):691–7.
Dulak J, Józkowicz A. Anti-angiogenic and anti-inflammatory effects of statins: relevance to anti-cancer therapy. Curr Cancer Drug Targets. 2005;5(8):579–94.
Sahebkar A, Rathouska J, Derosa G, Maffioli P, Nachtigal P. Statin impact on disease activity and C-reactive protein concentrations in systemic lupus erythematosus patients: a systematic review and meta-analysis of controlled trials. Autoimmun Rev. 2016;15(4):344–53.
Chruściel P, Sahebkar A, Rembek-Wieliczko M, Serban MC, Ursoniu S, Mikhailidis DP, et al. Impact of statin therapy on plasma adiponectin concentrations: A systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208.
Bianconi V, Sahebkar A, Banach M, Pirro M. Statins, haemostatic factors and thrombotic risk. Curr Opin Cardiol. 2017;32(4):460–6.
Sahebkar A, Serban C, Ursoniu S, Mikhailidis DP, Undas A, Lip GY, et al. The impact of statin therapy on plasma levels of von Willebrand factor antigen. Thromb Haemost. 2016;115(03):520–32.
Sahebkar A, Catena C, Ray KK, Vallejo-Vaz AJ, Reiner Ž, Sechi LA, et al. Impact of statin therapy on plasma levels of plasminogen activator inhibitor-1. Thromb Haemost. 2016;116(07):162–71.
Sahebkar A, Serban C, Mikhailidis DP, Undas A, Lip GY, Muntner P, et al. Association between statin use and plasma D-dimer levels. Thromb Haemost. 2015;114(09):546–57.
Kabaklić A, Fras Z. Moderate-dose atorvastatin improves arterial endothelial function in patients with angina pectoris and normal coronary angiogram: a pilot study. Arch Med Sci. 2017;13(4):827–36.
Katsiki N, Reiner Ž, Tedeschi Reiner E, Al-Rasadi K, Pirro M, Mikhailidis DP, et al. Improvement of endothelial function by pitavastatin: a meta-analysis. Expert Opin Pharmacother. 2018;19(3):279–86.
Sahebkar A, Kotani K, Serban C, Ursoniu S, Mikhailidis DP, Jones SR, et al. Statin therapy reduces plasma endothelin-1 concentrations: a meta-analysis of 15 randomized controlled trials. Atherosclerosis. 2015;241(2):433–42.
Derosa G, Maffioli P, Reiner Ž, Simental-Mendía LE, Sahebkar A. Impact of statin therapy on plasma uric acid concentrations: a systematic review and meta-analysis. Drugs. 2016;76(9):947–56.
Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the develo** world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. 2012;60(14):1207–16.
Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
Pouyan A, S, Erfanmanesh M. Is Interleukin-38 a key player cytokine in atherosclerosis immune gene therapy? Med Hypotheses. 2019;125:139–43.
Kovanen PT. Mast cells as potential accelerators of human atherosclerosis—from early to late lesions. Int J Mol Sci. 2019;20(18):4479.
Mohajeri M, Kovanen PT, Bianconi V, Pirro M, Cicero AF, Sahebkar A. Mast cell tryptase–marker and maker of cardiovascular diseases. Pharmacol Ther. 2019;199:91–110.
Wezel A, Lagraauw HM, van der Velden D, de Jager SC, Quax PH, Kuiper J, et al. Mast cells mediate neutrophil recruitment during atherosclerotic plaque progression. Atherosclerosis. 2015;241(2):289–96.
Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med. 2007;13(6):719–24.
Bot I (2007) Jager SCA de, Zernecke A, Lindstedt KA, Berkel TJC van, Weber C, et al. Perivascular mast cells promote atherogenesis and induce plaque destabilization in apolipoprotein Eedeficient mice Circulation 115:2516–2525.
Smith DD, Tan X, Raveendran VV, Tawfik O, Stechschulte DJ, Dileepan KN. Mast cell deficiency attenuates progression of atherosclerosis and hepatic steatosis in apolipoprotein E-null mice. Am J Phys Heart Circ Phys. 2012;302(12):H2612–21.
• Kritikou E, Depuydt MAC, de Vries MR, Mulder KE, Govaert AM, Smit MD, et al. Flow Cytometry-based characterization of mast cells in human atherosclerosis. Cells. 2019;8(4). https://doi.org/10.3390/cells8040334. This study revealed a strong relationship between the presence of IgE and mast cells activation in human atherosclerosis plaques.
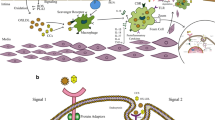
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11(5):373–84.
Lee CC, Avalos AM, Ploegh HL. Accessory molecules for toll-like receptors and their function. Nat Rev Immunol. 2012;12(3):168.
Verstrepen L, Bekaert T, Chau T-L, Tavernier J, Chariot A, Beyaert R. TLR-4, IL-1R and TNF-R signaling to NF-κB: variations on a common theme. Cell Mol Life Sci. 2008;65(19):2964–78.
Wijnand K, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010;209(2):314–20.
Pasterkamp G, Van Keulen J, De Kleijn D. Role of toll-like receptor 4 in the initiation and progression of atherosclerotic disease. Eur J Clin Investig. 2004;34(5):328–34.
Sandig H, Bulfone-Paus S. TLR signaling in mast cells: common and unique features. Front Immunol. 2012;3:185.
Bahrami A, Parsamanesh N, Atkin SL, Banach M, Sahbekar A. Effect of statins on toll-like receptors: a new insight to pleiotropic effects. Pharmacol Res, 2018
Gondokaryono SP, Ushio H, Niyonsaba F, Hara M, Takenaka H, Jayawardana ST, et al. The extra domain a of fibronectin stimulates murine mast cells via toll-like receptor 4. J Leukoc Biol. 2007;82(3):657–65.
Chansrichavala P, Chantharaksri U, Sritara P, Chaiyaroj SC. Atorvastatin attenuates TLR4-mediated NF-[kappa] B activation in a MyD88-dependent pathway. Asian Pac J Allergy Immunol. 2009;27(1):49–57.
Shen D-Z, **n S-L, Chen C, Liu T. Effect of atorvastatin on expression of TLR4 and NF-κB p65 in atherosclerotic rabbits. Asian Pac J Trop Med. 2013;6(6):493–6.
• Kapelouzou A, Giaglis S, Peroulis M, Katsimpoulas M, Moustardas P, Aravanis CV, et al. Overexpression of toll-like receptors 2, 3, 4, and 8 is correlated to the vascular atherosclerotic process in the hyperlipidemic rabbit model: the effect of statin treatment. J Vasc Res. 2017;54(3):156–69. Fluvastatin treatment significantly decreased the progression of atherosclerosis and reduced inflammation via toll-like receptors downregulation.
Albert MA, Glynn RJ, Wolfert RL, Ridker PM. The effect of statin therapy on lipoprotein associated phospholipase A2 levels. Atherosclerosis. 2005;182(1):193–8.
Magrioti V, Kokotos G. Phospholipase A2 inhibitors as potential therapeutic agents for the treatment of inflammatory diseases. Expert Opin Ther Pat. 2010;20(1):1–18.
Katan M, Moon YP, Paik MC, Wolfert RL, Sacco RL, Elkind MS. Lipoprotein-associated phospholipase A2 is associated with atherosclerotic stroke risk: the northern Manhattan study. PLoS One. 2014;9(1):e83393.
Tsimihodimos V, Karabina S-AP, Tambaki AP, Bairaktari E, Goudevenos JA, Chapman MJ, et al. Atorvastatin preferentially reduces LDL-associated platelet-activating factor acetylhydrolase activity in dyslipidemias of type IIA and type IIB. Arterioscler Thromb Vasc Biol. 2002;22(2):306–11.
Kleinegris MC, ten Cate-Hoek AJ, Ten Cate H. Coagulation and the vessel wall in thrombosis and atherosclerosis. Pol Arch Med Wewn. 2012;122(11):557–66.
Talreja J, Kabir MH, Filla B, M, Stechschulte DJ, Dileepan KN. Histamine induces toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to gram-positive and gram-negative bacterial cell wall components. Immunology. 2004;113(2):224–33.
Roche C, Trimble E, Ennis M. Effect of in vivo and in vitro lovastatin treatment on mast cell activation. Int Arch Allergy Immunol. 1995;108(3):240–6.
Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–30.
• Sahid MNA, Liu S, Kiyoi T, Maeyama K. Inhibition of the mevalonate pathway by simvastatin interferes with mast cell degranulation by disrupting the interaction between Rab27a and double C2 alpha proteins. Eur J Pharmacol. 2017;814:255–63. This study showed that simvastatin treatment suppress mast cell degranulation by inhibiting mevalonate pathway.
Kagami S-i, Kanari H, Suto A, Fujiwara M, Ikeda K, Hirose K, et al. HMG-CoA reductase inhibitor simvastatin inhibits proinflammatory cytokine production from murine mast cells. Int Arch Allergy Immunol. 2008;146(Suppl. 1):61–6.
Fujimoto M, Oka T, Murata T, Hori M, Ozaki H. Fluvastatin inhibits mast cell degranulation without changing the cytoplasmic Ca2+ level. Eur J Pharmacol. 2009;602(2–3):432–8.
Kolawole EM, McLeod JJA, Ndaw V, Abebayehu D, Barnstein BO, Faber T, et al. Fluvastatin suppresses mast cell and basophil IgE responses: genotype-dependent effects. J Immunol. 2016;196(4):1461–70.
Kolawole EM. The effect of fluvastatin on mast cell function: genotype dependence, 2014
Virgolini I, Li S-R, Yang Q, Koller E, Sperr WR, Angelberger MLP, et al. Characterization of LDL and VLDL binding sites on human basophils and mast cells. Arterioscler Thromb Vasc Biol. 1995;15(1):17–26.
Lindstedt K, Kokkonen J, Kovanen P. Soluble heparin proteoglycans released from stimulated mast cells induce uptake of low density lipoproteins by macrophages via scavenger receptor-mediated phagocytosis. J Lipid Res. 1992;33(1):65–75.
Li S, Dudczak R, Koller E, Baghestanian M, Ghannadan M, Minar E, et al. Effect of statins on lipoprotein receptor expression in cell lines from human mast cells and basophils. Eur J Clin Pharmacol. 2003;59(7):507–16.
Di Salvo E, Ventura-Spagnolo E, Casciaro M, Navarra M, Gangemi S. IL-33/IL-31 axis: a potential inflammatory pathway. Mediat Inflamm. 2018;2018.
Lu J, Kang J, Zhang C, Zhang X. The role of IL-33/ST2L signals in the immune cells. Immunol Lett. 2015;164(1):11–7.
Stankovic M, Ljujic B, Babic S, Maravic-Stojkovic V, Mitrovic S, Arsenijevic N, et al. IL-33/IL-33R in various types of carotid artery atherosclerotic lesions. Cytokine. 2019;120:242–50.
Moulin D, Donzé O, Talabot-Ayer D, Mézin F, Palmer G, Gabay C. Interleukin (IL)-33 induces the release of pro-inflammatory mediators by mast cells. Cytokine. 2007;40(3):216–25.
Rönnberg E, Ghaib A, Ceriol C, Enoksson M, Arock M, Säfholm J, Ekoff M, Nilsson G. Divergent effects of acute and prolonged interleukin 33 exposure on mast cell IgE-mediated functions. Frontiers in immunology 10. 2019
Hsu C-L, Neilsen CV, Bryce PJ. IL-33 is produced by mast cells and regulates IgE-dependent inflammation. PLoS One. 2010;5(8):e11944.
Saluja R, Hawro T, Eberle J, Church M, Maurer M. Interleukin-33 promotes the proliferation of mouse mast cells through ST2/MyD88 and p38 MAPK-dependent and kit-independent pathways. J Biol Regul Homeost Agents. 2014;28(4):575–85.
Taruselli M. The effect of statins on IL-33 mediated mast cell function. 2015
Heo SH, Cho C-H, Kim HO, Jo YH, Yoon K-S, Lee JH, et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: involvement of matrix metalloproteinases 2 and 9. J Clin Neurol. 2011;7(2):69–76.
Jager NA, De Vries BMW, Hillebrands J-L, Harlaar NJ, Tio RA, Slart RH, et al. Distribution of matrix metalloproteinases in human atherosclerotic carotid plaques and their production by smooth muscle cells and macrophage subsets. Mol Imaging Biol. 2016;18(2):283–91.
Kimata M, Ishizaki M, Tanaka H, Nagai H, Inagaki N. Production of matrix Metalloproteinases in human cultured mast cells: involvement of protein kinase C—mitogen activated protein kinase kinase—extracellular signal-regulated kinase pathway. Allergol Int. 2006;55(1):67–76.
Xu L, Cai Z, Yang F, Chen M. Activation-induced upregulation of MMP9 in mast cells is a positive feedback mediator for mast cell activation. Mol Med Rep. 2017;15(4):1759–64.
Silvello D, Narvaes LB, Albuquerque LC, Forgiarini LF, Meurer L, Martinelli NC, et al. Serum levels and polymorphisms of matrix metalloproteinases (MMPs) in carotid artery atherosclerosis: higher MMP-9 levels are associated with plaque vulnerability. Biomarkers. 2014;19(1):49–55.
Sai L, YanQiu Z, DongMei C, YinJun L. Effect of rosuvastatin and benazepril on matrix metalloproteinase-2, matrix metalloproteinase-9 and leukotriene B4 of patients with acute myocardial infarction. Trop J Pharm Res. 2019;18(3):625–30.
Fujimoto S, Hartung D, Ohshima S, Edwards DS, Zhou J, Yalamanchili P, et al. Molecular imaging of matrix metalloproteinase in atherosclerotic lesions: resolution with dietary modification and statin therapy. J Am Coll Cardiol. 2008;52(23):1847–57.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kouhpeikar, H., Delbari, Z., Sathyapalan, T. et al. The Effect of Statins through Mast Cells in the Pathophysiology of Atherosclerosis: a Review. Curr Atheroscler Rep 22, 19 (2020). https://doi.org/10.1007/s11883-020-00837-9
Published:
DOI: https://doi.org/10.1007/s11883-020-00837-9