Abstract
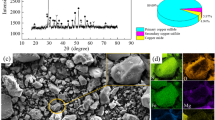
A new extraction technology of chlorination roasting with a mixture of MgCl2·6H2O and NaCl was investigated to co-extract nickel and copper from low-grade nickel ores. The effects of some key factors on the extraction of nickel, copper, and iron were studied, and the results show that,when the roasting temperature is 850°C, the roasting time is 1.5 h, the mass ratio of magnesium chloride hexahydrate to sodium chloride is 5:2, the mass ratio of ore to chlorination agent is 1:0.6, and the ore particle size is 180–200 mesh, the extraction of nickel and copper can reach 83.6% and 81.3%, respectively, but less than 3% of impurity iron enters the leach solution. XRD and SEM were used to analyze the mineral phase of the roasted products and leach residue to determine the phase transformation. The kinetics of the chlorination process were investigated by the Kissinger and Flynn–Wall–Ozawa method to determine the average activation energy and the kinetic equation.






Similar content being viewed by others
References
A.F. Sahayaraj, J. Venkatesh, R. Raghu, R.S. Kumar, and R. Subbiah, Mater Today Proc (2021).
R.A. Hussain, and I. Hussain, J Solid State Chem 277, 316. (2019).
J.E. Kanyo, S. Schaffner, R.S. Uwanyuze, and K.S. Leary, J Eur Ceram Soc 40, 4955. (2020).
S.M.A. Shibli, G.J. Harikrishnan, V.R. Anupama, K.S. Chinchu, and B.N. Meena, Surf Coat Technol 262, 48. (2015).
M. Liu, S. Su, Y. Yao, X. Wu, N. Cai, and Q. Guan, Acta Petrolog Sin 36, 1151. (2020).
I.A. Dourgham, K.M. Fawzy, and H.E. Frimmel, Arab J Geosci 10, 1. (2017).
E. Pakostova, B.M. Grail, and D.B. Johnson, Miner Eng 106, 102. (2017).
S. Li, H. Zhong, Y. Hu, J. Zhao, Z. He, and G. Gu, Biores Technol 153, 300. (2014).
S. He, J. Wang, and J. Yan, Hydrometallurgy 104, 235. (2010).
S. He, C. Liao, X. Wang, and J. Wang, Min Metall Explor 37, 433. (2020).
A. Kritskii, K. Karimov, and S. Naboichenko, Solid State Phenom 299, 1052. (2020).
W. **ao, X. Liu, and Z. Zhao, Hydrometallurgy 194, 105353. (2020).
N. Safitri, M.Z. Mubarok, I.U. Meidji, J. Hardi, and H. Jayadi, J Phys Conf Ser 1763, 012044. (2021).
N. Picazo-Rodríguez, D. Ma, J. Soria-Aguilar, A. Martínez-Luévanos, and F.R. Carrillo-Pedroza, Miner Eng 10, 1. (2020).
Z. Moravvej, A. Mohebbi, and S. Daneshpajouh, Mater Manuf Process 33, 1. (2018).
F. Cui, W. Mu, Y. Zhai, and X. Guo, Russ J Non-Ferr Met 61, 27. (2020).
Q. Sun, H. Cheng, X. Mei, Y. Liu, and X. Lu, Sci Rep 10, 9916. (2020).
W. Mu, F. Cui, Z. Huang, Y. Zhai, X. Qian, and S. Luo, J Clean Prod 177, 371. (2018).
F. Cui, W. Mu, S. Wang, H. **n, Q. Xu, Y. Zhai, and S. Luo, Miner Eng 128, 104. (2018).
W. Mu, Z. Huang, H. **n, S. Luo, and Q. Xu, JOM 71, 4647. (2019).
P.V. Aleksandrov, A.S. Medvedev, M.F. Milovanov, V.A. Imideev, S.A. Kotova, and D.O. Moskovskikh, Int J Miner Process 161, 13. (2017).
C. Xu, H.W. Cheng, G.S. Li, C.Y. Lu, X.G. Lu, X.L. Zou, and Q. Xu, Int J Miner Metall Mater 24, 377. (2017).
F. Cui, W. Mu, W. Shuai, H. **n, H. Shen, X. Qian, Y. Zhai, and S. Luo, Sep Purif Technol 195, 149. (2018).
W. Mu, F. Cui, H. **n, Y. Zhai, and Q. Xu, Hydrometallurgy 191, 105187. (2020).
F. Cui, W. Mu, Y. Zhai, and X. Guo, Sep Purif Technol 239, 116577. (2020).
X. Song, G. Liu, Z. Sun, and J. Yu, Asia-Pac J Chem Eng 7, 221. (2012).
H.E. Kissinger, Anal Chem 29, 1702. (1957).
S. Vyazovkin, Molecules 25, 1. (2020).
J.H. Flynn, and L.A. Wall, J Polym Sci Part B 4, 323. (1966).
T. Ozawa, Bull Chem Soc Jpn 38, 1881. (1965).
Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 52074069), the Natural Science Foundation of Hebei Province (No. E2020501022), the Science and Technology Project of Hebei Education Department (No. ZD2021331) and the Fundamental Research Funds for the Central Universities (No. N182304016) for the financially supported of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xu, X., Mu, W., Wang, L. et al. Direct Extraction of Nickel and Copper from Low-Grade Nickel Sulfide Ore by Chlorination Roasting with Mixed MgCl2·6H2O and NaCl. JOM 74, 1989–1999 (2022). https://doi.org/10.1007/s11837-021-05122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-05122-x