Abstract
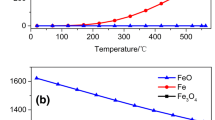
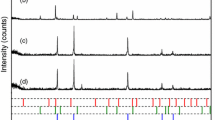
The mechanisms for magnetizing roasting of Fe2O3 into Fe3O4 under microwave heating and electrical heating have been studied through thermogravimetric analyses, x-ray diffraction (XRD) measurements, and reaction kinetic calculations. In the reduction process, activated carbon was used as a reducing agent and argon as the protective gas. The results of heating tests indicated that the temperature heating speed during microwave heating is 50 times faster than that of electrical heating. The maximum conversion ratio of Fe2O3 by microwave heating is 24.5% higher than that by electrical heating. XRD results showed that the required temperature for Fe2O3 to completely convert into Fe3O4 by microwave heating is 200°C lower than that by electrical heating. Reaction kinetics parameters calculation results showed that the controlling step of microwave magnetizing is a phase boundary reaction of the contracted ball at 250–450°C with an apparent activation energy of 45 kJ/mol, whereas the controlling step of electrical magnetizing is a chemical reaction of stochastic coring at 450–650°C with an apparent activation energy of 225 kJ/mol.








Similar content being viewed by others
References
K.E. Haque, Int. J. Miner. Process. 57, 1 (2009).
C.A. Pickles, Miner. Eng. 22, 1102 (2009).
C.A. Pickles, Miner. Eng. 22, 1112 (2009).
N. Standish and H. Womer, J. Microw. Power E 25, 177 (1990).
S. Zhong, H.E. Goetzman, and R.L. Bleifuss, Miner. Metall. Proc. 13, 174 (1996).
E. Lester, S. Kingman, C. Dodds, and J. Patrick, Fuel 85, 2057 (2006).
M. Samouhos, M. Taxiarchou, P.E. Tsakiridis, and K. Potiriadis, J. Hazard. Mater. 254, 193 (2013).
C.A. Crane, M.L. Pantoya, B.L. Weeks, and M. Saed, Powder Technol. 256, 113 (2014).
S. Zhang, J. Peng, and M. Huang, Chin. J. Rare Met. 30, 78 (2006).
J. Chen, L. Liu, J. Zeng, R. Ren, and J. Liu, Iron Steel 39, 1 (2004).
K. Ishizaki and K. Nagata, ISIJ Int. 48, 1159 (2008).
Y. Lei, Y. Li, and J. Peng, ISIJ Int. 51, 337 (2011).
M. Sato, A. Matsubara, S. Takayama, S. Sudo, O. Motojima, K. Nagata, K. Ishizaki, T. Hayashi, D. Agrawal, and R. Roy, Advanced Processing of Metals and Materials (Sohn International Symposium), Volume 5, New, Improved and Existing Technologies: Iron and Steel, Recycling and Waste Treatment, eds. F. Kongoli and R.G. Reddy (Warrendale, PA: TMS, 2006), pp. 157–170.
J. Li, B. Zhang, and B. Li, Metal Mine 47, 89 (2010).
J. Li, B. Li, and B. Zhang, J. Univ. Sci. Technol. B 33, 1127 (2011).
T.L. Zhang, R.Z. Hu, and F.P. Li, Thermochim. Acta 244, 177 (1994).
Y. Sun, P. Gao, Y. Han, and D. Ren, Ind. Eng. Chem. Res. 52, 2323 (2013).
R. Hu and Q. Shi, Thermal Analysis Kinetics, 2nd ed. (Bei**g, China: Science Press, 2001).
Acknowledgements
Financial supports from Inner Mongolia Natural Science Foundation (No. 2012MS0714), Inner Mongolia scientific research project of higher educational institutes (NJZY13149), and the special funds of the Key Laboratory of Integrated Exploitation of Bayan Obo Multi-Metal Resources of Inner Mongolia University of Science and Technology (BO-13-001) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Li, B., Han, J. et al. A Comparative Study on the Reduction Mechanism of Fe2O3 Under Different Heating Methods. JOM 66, 1529–1536 (2014). https://doi.org/10.1007/s11837-014-1083-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-014-1083-z