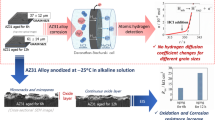
Abstract
Biodegradable Mg alloys have generated great interest for biomedical applications. Accurate predictions of in vivo degradation of Mg alloys through cost-effective in vivo evaluations require the latter to be conducted in an environment close to that of physiological scenarios. However, the roles of glucose and buffering agents in regulating the in vivo degradation performance of Mg alloys has not been elucidated. Herein, degradation behavior of AZ31 alloy is investigated by hydrogen evolution measurements, pH monitoring and electrochemical tests. Results indicate that glucose plays a content-dependent role in degradation of AZ31 alloy in buffer-free saline solution. The presence of a low concentration of glucose, i.e. 1.0 g/L, decreases the corrosion rate of Mg alloy AZ31, whereas the presence of 2.0 and 3.0 g/L glucose accelerates the corrosion rate during long term immersion in saline solution. In terms of Tris-buffered saline solution, the addition of glucose increases pH value and promotes pitting corrosion or general corrosion of AZ31 alloy. This study provides a novel perspective to understand the bio-corrosion of Mg alloys in buffering agents and glucose containing solutions.
Similar content being viewed by others
References
Zheng Y F, Gu X N, Witte F. Biodegradable metals. Materials Science and Engineering R: Reports, 2014, 77(2): 1–34
Zeng R C, Qi WC, Cui H Z, et al. In vitro corrosion of as-extruded Mg–Ca alloys — The influence of Ca concentration. Corrosion Science, 2015, 96: 23–31
Witte F, Kaese V, Haferkamp H, et al. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials, 2005, 26(17): 3557–3563
Ascencio M, Pekguleryuz M, Omanovic S. An investigation of the corrosion mechanisms of WE43Mg alloy in a modified simulated body fluid solution: The effect of electrolyte renewal. Corrosion Science, 2015, 91(5): 297–310
Li Y, Cai S, Xu G, et al. Synthesis and characterization of a phytic acid/mesoporous 45S5 bioglass composite coating on a magnesium alloy and degradation behavior. RSC Advances, 2015, 5(33): 25708–25716
Zeng R C, Cui L Y, Jiang K, et al. In vitro corrosion and cytocompatibility of a microarc oxidation coating and poly(L-lactic acid) composite coating on Mg–1Li–1Ca alloy for orthopedic implants. ACS Applied Materials & Interfaces, 2016, 8(15): 10014–10028
Gu X N, Zhou WR, Zheng Y F, et al. Corrosion fatigue behaviors of two biomedical Mg alloys–AZ91D and WE43–in simulated body fluid. Acta Biomaterialia, 2010, 6(12): 4605–4613
** W, Wu G, Feng H, et al. Improvement of corrosion resistance and biocompatibility of rare-earth WE43 magnesium alloy by neodymium self-ion implantation. Corrosion Science, 2015, 94 (Supplement C): 142–155
Liu X, Yang Q, Li Z, et al. A combined coating strategy based on atomic layer deposition for enhancement of corrosion resistance of AZ31 magnesium alloy. Applied Surface Science, 2018, 434: 1101–1111
Walter R, Kannan MB, He Y, et al. Effect of surface roughness on the in vitro degradation behaviour of a biodegradable magnesiumbased alloy. Applied Surface Science, 2013, 279(Supplement C): 343–348
Cipriano A F, Sallee A, Tayoba M, et al. Cytocompatibility and early inflammatory response of human endothelial cells in direct culture with Mg–Zn–Sr alloys. Acta Biomaterialia, 2017, 48 (Supplement C): 499–520
Shi Y, Zhang L, Chen J, et al. In vitro and in vivo degradation of rapamycin-eluting Mg–Nd–Zn–Zr alloy stents in porcine coronary arteries. Materials Science and Engineering C, 2017, 80(Supplement C): 1–6
Chang W H, Qu B, Liao A D, et al. In vitro biocompatibility and antibacterial behavior of anodic coatings fabricated in an organic phosphate containing solution on Mg–1.0Ca alloys. Surface and Coatings Technology, 2016, 289: 75–84
Wang X J, Xu D K, Wu R Z, et al. What is going on in magnesium alloys? Journal of Materials Science and Technology, 2018, 34(2): 245–247
Wang L, Shinohara T, Zhang B P. Influence of chloride, sulfateand bicarbonate anions on the corrosion behavior of AZ31 magnesium alloy. Journal of Alloys and Compounds, 2010, 496 (1–2): 500–507
Cipriano A F, Sallee A, Guan R G, et al. A comparison study on the degradation and cytocompatibility of Mg–4Zn–xSr alloys in direct culture. ACS Biomaterials Science & Engineering, 2017, 3 (4): 540–550
**n Y, Hu T, Chu P K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: a review. Acta Biomaterialia, 2011, 7(4): 1452–1459
Zhen Z, Zheng Y, Ge Z, et al. Biological effect and molecular mechanism study of biomaterials based on proteomic research. Journal of Materials Science and Technology, 2017, 33(7): 607–615
Cui L Y, Sun L, Zeng R C, et al. In vitro degradation and biocompatibility of Mg–Li–Ca alloys — The influence of Li content. Science China Materials, 2018, 61(4): 607–618
Wang J L, Mukherjee S, Nisbet D R, et al. In vitro evaluation of biodegradable magnesium alloys containing micro-alloying additions of strontium, with and without zinc. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2015, 3(45): 8874–8883
Hou L, Li Z, Zhao H, et al. Microstructure, mechanical properties, corrosion behavior and biocompatibility of as-extruded biodegradable Mg–3Sn–1Zn–0.5Mn alloy. Journal of Materials Science and Technology, 2016, 32(9): 874–882
Li C Q, Xu D K, Yu S, et al. Effect of icosahedral phase on crystallographic texture and mechanical anisotropy of Mg–4%Li based alloys. Journal of Materials Science and Technology, 2017, 33(5): 475–480
Jia H, Feng X, Yang Y. Microstructure and corrosion resistance of directionally solidified Mg–2 wt.% Zn alloy. Corrosion Science, 2017, 120: 75–81
Zeng R C, Qi WC, Cui H Z, et al. In vitro corrosion of as-extruded Mg–Ca alloys — The influence of Ca concentration. Corrosion Science, 2015, 96: 23–31
Jiang H, Li F, Zeng X. Microstructural characteristics and deformation of magnesium alloy AZ31 produced by continuous variable cross-section direct extrusion. Journal of Materials Science and Technology, 2017, 33(6): 573–579
Li C Q, Xu D K, Wang B J, et al. Suppressing effect of heat treatment on the Portevin–Le Chatelier phenomenon of Mg–4% Li–6%Zn–1.2%Y alloy. Journal of Materials Science and Technology, 2016, 32(12): 1232–1238
Feng H, Liu S, Du Y, et al. Effect of the second phases on corrosion behavior of the Mg–Al–Zn alloys. Journal of Alloys and Compounds, 2017, 695: 2330–2338
Jia Z, **ong P, Shi Y, et al. Inhibitor encapsulated, self-healable and cytocompatible chitosan multilayer coating on biodegradable Mg alloy: a pH-responsive design. Journal of Materials Chemistry B: Materials for Biology and Medicine, 2016, 4(14): 2498–2511
Cui L Y, Zeng R C, Zhu X X, et al. Corrosion resistance of biodegradable polymeric layer-by-layer coatings on magnesium alloy AZ31. Frontiers of Materials Science, 2016, 10(2): 134–146
Chen J Y, Chen X B, Li J L, et al. Electrosprayed PLGA smart containers for active anti-corrosion coating on magnesium alloy AMlite. Journal of Materials Chemistry A: Materials for Energy and Sustainability, 2014, 2(16): 5738–5743
Wan P, Tan L, Yang K. Surface modification on biodegradable magnesium alloys as orthopedic implant materials to improve the bio-adaptability: A review. Journal of Materials Science and Technology, 2016, 32(9): 827–834
Merino M C, Pardo A, Arrabal R, et al. Influence of chloride ion concentration and temperature on the corrosion of Mg–Al alloys in salt fog. Corrosion Science, 2010, 52(5): 1696–1704
Cui L Y, Gao S D, Li P P, et al. Corrosion resistance of a selfhealing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corrosion Science, 2017, 118: 84–95
Song Y, Shan D, Han E H. Pitting corrosion of a Rare Earth Mg alloy GW93. Journal of Materials Science and Technology, 2017, 33(9): 954–960
Wen C L, Guan S K, Peng L, et al. Characterization and degradation behavior of AZ31 alloy surface modified by bone-like hydroxyapatite for implant applications. Applied Surface Science, 2009, 255(13–14): 6433–6438
Zeng R C, Hu Y, Guan S K, et al. Corrosion of magnesium alloy AZ31: The influence of bicarbonate, sulphate, hydrogen phosphate and dihydrogen phosphate ions in saline solution. Corrosion Science, 2014, 86(10): 171–182
Walker J, Shadanbaz S, Kirkland N T, et al. Magnesium alloys: Predicting in vivo corrosion with in vitro immersion testing. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2012, 100(4): 1134–1141
Guo L, Zhang F, Song L, et al. Corrosion resistance of ceria/ polymethyltrimethoxysilane modified magnesium hydroxide coating on AZ31 magnesium alloy. Surface and Coatings Technology, 2017, 328: 121–133
Sales C H, Pedrosa L F, Lima J G, et al. Influence of magnesium status and magnesium intake on the blood glucose control in patients with type 2 diabetes. Clinical Nutrition, 2011, 30(3): 359–364
Hruby A, Meigs J B, O’Donnell C J, et al. Higher magnesium intake reduces risk of impaired glucose and insulin metabolism and progression from prediabetes to diabetes in middle-aged Americans. American Journal of Diseases of Children, 2014, 37 (2): 419–427
Zeng R C, Li X T, Li S Q, et al. In vitro degradation of pure Mg in response to glucose. Scientific Reports, 2015, 5(1): 13026
Cui L Y, Li X T, Zeng R C, et al. In vitro corrosion of Mg–Ca alloy — The influence of glucose content. Frontiers of Materials Science, 2017, 11(3): 284–295
Wang Y, Cui L Y, Zeng R C, et al. In vitro degradation of pure magnesium—the effects of glucose and/or amino acid. Materials, 2017, 10(7): 725
Kannan M B, Khakbaz H, Yamamoto A. Understanding the influence of HEPES buffer concentration on the biodegradation of pure magnesium: An electrochemical study. Materials Chemistry and Physics, 2017, 197: 47–56
Cui L Y, Hu Y, Zeng R C, et al. New insights into the effect of Tris-HCl and Tris on corrosion of magnesium alloy in presence of bicarbonate, sulfate, hydrogen phosphate and dihydrogen phosphate ions. Journal of Materials Science and Technology, 2017, 33 (9): 971–986
Kirkland N T, Waterman J, Birbilis N, et al. Buffer-regulated biocorrosion of pure magnesium. Journal of Materials Science: Materials in Medicine, 2012, 23(2): 283–291
**n Y, Chu P K. Influence of Tris in simulated body fluid on degradation behavior of pure magnesium. Materials Chemistry and Physics, 2010, 124(1): 33–35
Zeng R C, Cui L Y, Jiang K, et al. In vitro corrosion and cytocompatibility of a microarc oxidation coating and poly(Llactic acid) composite coating on Mg–1Li–1Ca alloy for orthopedic implants. ACS Applied Materials & Interfaces, 2016, 8(15): 10014–10028
Zhao Y B, Liu H P, Li C Y, et al. Corrosion resistance and adhesion strength of a spin-assisted layer-by-layer assembled coating on AZ31 magnesium alloy. Applied Surface Science, 2018, 434: 787–795
Lin X, Tan L, Zhang Q, et al. The in vitro degradation process and biocompatibility of a ZK60 magnesium alloy with a forsteritecontaining micro-arc oxidation coating. Acta Biomaterialia, 2013, 9(10): 8631–8642
Cui Z, Li X, **ao K, et al. Atmospheric corrosion of field-exposed AZ31 magnesium in a tropical marine environment. Corrosion Science, 2013, 76(Supplement C): 243–256
Zeng R C, Li X T, Liu L J, et al. In vitro degradation of pure Mg for esophageal stent in artificial saliva. Journal of Materials Science and Technology, 2016, 32(5): 437–444
Zong Y, Yuan G, Zhang X, et al. Comparison of biodegradable behaviors of AZ31 and Mg–Nd–Zn–Zr alloys in Hank’s physiological solution. Materials Science and Engineering B, 2012, 177(5): 395–401
Shahabi-Navid M, Esmaily M, Svensson J E, et al. NaCl-induced atmospheric corrosion of the MgAl alloy AM50 — The influence of CO2. Clinical and Experimental Immunology, 2014, 161(6): C277–C287
Esmaily M, Shahabi-Navid M, Svensson J E, et al. Influence of temperature on the atmospheric corrosion of the Mg–Al alloy AM50. Corrosion Science, 2015, 90(Supplement C): 420–433
Rashidian M, Fattahi A. Comparison of thermochemistry of aspartame (artificial sweetener) and glucose. Carbohydrate Research, 2009, 344(1): 127–133
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos. 51571134 and 51601108), the SDUST Research Fund (2014TDJH104) and the Science and Technology Innovation Fund of SDUST for graduate students (SDKDYC180371).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, LY., Liu, B., Zeng, RC. et al. In vitro corrosion of magnesium alloy AZ31 — a synergetic influence of glucose and Tris. Front. Mater. Sci. 12, 184–197 (2018). https://doi.org/10.1007/s11706-018-0424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11706-018-0424-1