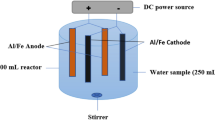
Abstract
The mineral industry consumes a large amount of xanthate as collector, especially for the sulfide mineral’s flotation. Nonetheless, the significant amount of xanthate also discharged from the processing plants, in proportion to the amount of used xanthate. In this work, the hazardous aqueous xanthate was aimed to be removed from water using electrocoagulation with three different electrodes: aluminum, iron and copper. Three separate experimental setups and designs were formed. The response surface methodology was implemented for the estimation of mathematical models for each electrode used in the electrocoagulation. All models were found statistically significant with p-values < 0.0015 and adjusted R2-values > 0.95. According to the formed linear and quadratic models, the commonly used aluminum electrodes in the literature proved to be useless for the elimination of xanthate. The dissolved aluminum could not react with xanthate, and no precipitation was observed during the treatment. Iron electrode yielded better results than aluminum when the system parameters were statistically optimized at pH 6.54 and electrical current of 0.6 A. The most effective formation of hydroxyl ferric xanthates was actualized at these specific levels of parameters, and the maximum removal% of xanthate was obtained as 82.34%. On the other hand, the last electrode made from copper remarkably decreased the concentration of xanthate with a removal% of 100%. The copper ions released from the electrodes with the help of electricity had a great affinity for xanthate. Following the electrocoagulation process, Cu(I) ethyl xanthate/Cu(II) ethyl xanthate efficiently precipitated, forming a yellow solid sediment at the bottom of the reactor. Consequently, the maximum desirability values for the removal of xanthate were found as 0.0037, 0.7700 and 1.000 respectively for aluminum, iron and copper electrodes. Based on this statistical optimization, the copper electrodes enabled the accomplished separation of hazardous xanthate from water using electrocoagulation at pH 9 and electrical current of 0.6 A.












Similar content being viewed by others
References
Ackerman PK, Harris GH, Klimpel RR, Aplan FF (1987) Evaluation of flotation collectors for copper sulfides and pyrite, I. Common sulfhydryl collectors. Int J Miner Process 21(1–2):105–127
Agorhom EA, Skinner W, Zanin M (2014) Diethylenetriamine depression of Cu-activated pyrite hydrophobised by xanthate. Miner Eng 57:36–42
Aitbara A, Cherifi M, Hazourli S, Leclerc JP (2016) Continuous treatment of industrial dairy effluent by electrocoagulation using aluminum electrodes. Desalin Water Treat 57(8):3395–3404
Akansha J, Nidheesh PV, Gopinath A, Anupama KV, Kumar MS (2020) Treatment of dairy industry wastewater by combined aerated electrocoagulation and phytoremediation process. Chemosphere 253:126652
Amin MI, Gado HS, Youssef WM, Masoud AM (2019) Precipitation of iron from wet process phosphoric acid using oxalic acid and potassium hexyl xanthate (PHX). Chem Pap 73(8):1871–1877
Amrollahi A, Massinaei M, Moghaddam AZ (2019) Removal of the residual xanthate from flotation plant tailings using bentonite modified by magnetic nano-particles. Miner Eng 134:142–155
Balouchi H, Baziar M, Dehghan A, Alidadi H, Shams M (2020) Combination of electrocoagulation and MOF adsorption systems for EBT removal from water. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1737035
Bertillas U, Björklund I, Borg H, Hörnström E (1985) Biologiska effekter av xantater. Naturvårdsverket rapport 3112
Bertsch PM, Parker DR (1996) Aqueous polynuclear aluminum species. Environ Chem Alum 2:117–168
Bhattacharya S (2021) Central composite design for response surface methodology and its application in pharmacy. In: Response surface methodology in engineering science. IntechOpen
Boening DW (1998) Aquatic toxicology and environmental fate of xanthates. Miner Eng 50:65–68
Bowden JL, Young CA (2016) Xanthate chemisorption at copper and chalcopyrite surfaces. J S Afr Inst Min Metall 116(6):503–508
Bu X, Chen F, Chen W, Ding Y (2019) The effect of whey protein on the surface property of the copper-activated marmatite in xanthate flotation system. Appl Surf Sci 479:303–310
Chang YK, Chang JE, Lin TT, Hsu YM (2002) Integrated copper-containing wastewater treatment using xanthate process. J Hazard Mater 94(1):89–99
Chen Y, Liu X, Chen J (2021) Steric hindrance effect on adsorption of xanthate on sphalerite surface: a DFT study. Miner Eng 165:106834
DeMartino AW, Zigler DF, Fukuto JM, Ford PC (2017) Carbon disulfide: Just toxic or also bioregulatory and/or therapeutic? Chem Soc Rev 46(1):21–39
Deng Z, Cheng W, Tang Y, Tong X, Liu Z (2021) Adsorption mechanism of copper xanthate on pyrite surfaces. Physicochem Probl Miner Process 57:46–60
Farrokhpay S (2011) The significance of froth stability in mineral flotation: a review. Adv Colloid Interface Sci 166(1–2):1–7
Fornasiero D, Montalti M, Ralston J (1995) Kinetics of adsorption of ethyl xanthate on pyrrhotite: in situ UV and infrared spectroscopic studies. J Colloid Interface Sci 172(2):467–478. https://doi.org/10.1006/jcis.1995.1277
Grano SR, Prestidge CA, Ralston J (1997) Solution interaction of ethyl xanthate and sulphite and its effect on galena flotation and xanthate adsorption. Int J Miner Process 52(2–3):161–186. https://doi.org/10.1016/s0301-7516(97)00066-5
Hao F, Davey KJ, Bruckard WJ, Woodcock JT (2008) Online analysis for xanthate in laboratory flotation pulps with a UV monitor. Int J Miner Process 89(1–4):71–75. https://doi.org/10.1016/j.minpro.2008.07.004
Harjanto S, Haryono D, Nugraha H, Saputra G, Baidillah MR, Al Huda M, Taruno WP (2021) Gas holdup and bubble flow transition characteristics in column flotation process determined by capacitive signals measurement. Braz J Chem Eng 38:361–372
Hu C, Wang S, Sun J, Liu H, Qu J (2016) An effective method for improving electrocoagulation process: optimization of Al13 polymer formation. Colloids Surf A Physicochem Eng Asp 489:234–240
Kang W, Hu J (2017) Influence of the carbon atom numbers and the structure of collectors on the coal fines flotation. Cogent Eng 4(1):1323374
Karimifard S, Moghaddam MRA (2018) Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review. Sci Total Environ 640:772–797
Kydros KA, Gallios GP, Matis KA (1994) Electrolytic flotation of pyrite. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 59(3):223–232
Mu Y, Peng Y, Lauten RA (2015) Electrochemistry aspects of pyrite in the presence of potassium amyl xanthate and a lignosulfonate-based biopolymer depressant. Electrochim Acta 174:133–142
Nasseri S, Yaqubov A, Alemi A, Nuriev A (2020) Optimization of copper and zinc ions removal from aqueous solution by modified nano-bentonite using response surface methodology. J Ultrafine Grained Nanostruct Mater 53(1):78–90
Noirant G, Benzaazoua M, Kongolo M, Bussière B, Frenette K (2019) Alternatives to xanthate collectors for the desulphurization of ores and tailings: pyrite surface chemistry. Colloids Surf A Physicochem Eng Asp 577:333–346
Nyangi MJ (2021) Remediation of arsenic from water using iron and aluminum electrodes in electrocoagulation technology: adsorption isotherm and kinetic studies. Chem Afr 4(4):943–954
Palacios RJS, Kim DG, Ko SO (2016) Humic acid removal by electrocoagulation: characterization of aluminum species and humic acid. Desalin Water Treat 57(24):10969–10979
Prestidge CA, Thiel AG, Ralston J, Smart RSC (1994) The interaction of ethyl xanthate with copper(II)-activated zinc sulphide: Kinetic effects. Colloids Surf A Physicochem Eng Asp 85(1):51–68. https://doi.org/10.1016/0927-7757(94)02748-x
Rezaei R, Massinaei M, Moghaddam AZ (2018) Removal of the residual xanthate from flotation plant tailings using modified bentonite. Miner Eng 119:1–10
Saber WI, El-Naggar NEA, El-Hersh MS, El-Khateeb AY, Elsayed A, Eldadamony NM, Ghoniem AA (2021) Rotatable central composite design versus artificial neural network for modeling biosorption of Cr 6+ by the immobilized Pseudomonas alcaliphila NEWG-2. Sci Rep 11(1):1–15
Safwat SM, Hamed A, Rozaik E (2019) Electrocoagulation/electroflotation of real printing wastewater using copper electrodes: a comparative study with aluminum electrodes. Sep Sci Technol 54(1):183–194
Salarirada MM, Behnamfardb A, Veglioc F (2021) Removal of xanthate from aqueous solutions by adsorption onto untreated and acid/base treated activated carbons. Desalin Water Treat 212:220–233
Scendo M (2005) Potassium ethyl xanthate as corrosion inhibitor for copper in acidic chloride solutions. Corros Sci 47(7):1738–1749
Shah M, Sarkar A, Mandal S (2021) Wastewater treatment. Elsevier Gezondheidszorg
Sharma K, Goswami M, Shadab M, Sarma NS, Devi A (2021) Treatment of paper mill effluent via electrochemical reaction and assessment of antibacterial activity of ZnO nanoparticles in in-vitro conditions. Chem Pap 75:3921–3929
Sheikh N, Leja J (1977) Msssbauer Spectroscopy of Fe-Xanthates Sep Sci 12(5):529–540
Sheikh N (1972) The chemical stability of the heavy metal xanthates. Ph.D. thesis, University of British Columbia, Canada
Shen Y, Nagaraj DR, Farinato R, Somasundaran P (2016) Study of xanthate decomposition in aqueous solutions. Miner Eng 93:10–15
Shen Y, Zhou P, Zhao S, Li A, Chen Y, Bai J, Han C, Wei D, Ao Y (2020) Synthesis of high-efficient TiO2/clinoptilolite photocatalyst for complete degradation of xanthate. Miner Eng 159:106640
Sillanpaa M (2020) Advanced water treatment: electrochemical methods, 1st edn. Elsevier, Amsterdam
Souto RM, Fox V, Laz MM, Perez M, González S (1996) Some experiments regarding the corrosion inhibition of copper by benzotriazole and potassium ethyl xanthate. J Electroanal Chem 411(1–2):161–165
Sun Z, Forsling W (1997) The degradation kinetics of ethyl-xanthate as a function of pH in aqueous solution. Miner Eng 10(4):389–400. https://doi.org/10.1016/s0892-6875(97)00016-2
Sürme Y, Demirci OB (2014) Determination of direct violet 51 dye in water based on its decolorisation by electrochemical treatment. Chem Pap 68(11):1491–1497
Voigt S, Szargan R, Suoninen E (1994) Interaction of copper (II) ions with pyrite and its influence on ethyl xanthate adsorption. Surf Interface Analy 21(8):526–536
Vučinić DR, Lazić PM, Rosić AA (2006) Ethyl xanthate adsorption and adsorption kinetics on lead-modified galena and sphalerite under flotation conditions. Colloids Surf A Physicochem Eng Asp 279(1–3):96–104
Wang X, Eric Forssberg KS, Bolin NJ (1989) Thermodynamic calculations on iron-containing sulphide mineral flotation systems, I. The stability of iron-xanthates. Int J Miner Process 27(1–2):1–19. https://doi.org/10.1016/0301-7516(89)90002-1
Wang H, Wen S, Han G, Feng Q (2019) Effect of copper ions on surface properties of ZnSO4-depressed sphalerite and its response to flotation. Sep Purif Technol 228:115756
**a L, Hart B, Larachi F, Gravel O (2019) Galvanic interaction of pyrite with Cu activated sphalerite and its effect on xanthate adsorption. Can J Chem Eng 97(10):2671–2677
Xu Y, Lay JP, Korte F (1988) Fate and effects of xanthates in laboratory freshwater systems. Bull Environ Contam Toxicol 41(5):683–689
Zini LP, Longhi M, Jonko E, Giovanela M (2020) Treatment of automotive industry wastewater by electrocoagulation using commercial aluminum electrodes. Process Saf Environ Prot 142:272–284
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
About this article
Cite this article
Çırak, M. Performance evaluation of electrocoagulation using aluminum, iron and copper electrodes for removal of xanthate. Chem. Pap. 76, 3661–3677 (2022). https://doi.org/10.1007/s11696-022-02120-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-022-02120-4