Abstract
Background
One of the new current treatment options for Diabetes Mellitus is about increasing glucagon-like peptide-1 (GLP-1) activity. GLP-1 with its incretin effect showed major role in glucose homeostasis. Gastroileostomy can increase GLP-1 secretion by rapid delivery of undigested food to the terminal ileum. We studied the early effects of a gastroileostomy on serum levels of GLP-1, glucose, and insulin in rats.
Methods
Gastroileostomies with side-to-side anastomosis were performed on 15 male New Zealand rats. Blood samples were obtained before and 1 week after the gastroileostomy.
Results
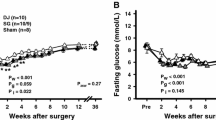
Our results showed that the rats lost a lot of weight from start (330 ± 15 g) to the end (240 ± 25 g) of the experiment (p = 0.048). The data analysis showed that the gastroileostomy surgery elevates the level of GLP-1in plasma significantly (89.1852 vs. 177.440 respectively; p < 0.001) and caused a significant decrease in plasma glucose as well (92.00 and 66.29 mg/dL respectively; p < 0.001). However, the insulin state elevated after the surgery significantly (8.03 vs. 9.89; p < 0.001).
Conclusion
In this study, we showed the effectiveness of gastroileostomy treatment to decrease body weight and plasma glucose with increased GLP-1 in rats. This small rat model suggests the potential of this surgery to treat type 2 diabetes mellitus.




Similar content being viewed by others
References
Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53.
Dupre J, Behme MT, McDonald TJ. Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab. 2004;89(7):3469–73.
Shyangdan DS, Royle P, Clar C, et al. Glucagon-like peptide analogues for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2011;10:CD006423.
Lee YS, Jun HS. Anti-diabetic actions of glucagon-like peptide-1 on pancreatic beta-cells. Metabolism. 2014;63(1):9–19.
Koliaki C, Doupis J. Incretin-based therapy: a powerful and promising weapon in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2011;2(2):101–21.
Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741–4.
Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–6.
Vilsboll T, Krarup T, Deacon CF, et al. Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes. 2001;50(3):609–13.
Stoffers DA, Desai BM, DeLeon DD, et al. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes. 2003;52(3):734–40.
Parkes DJC, Smith P, et al. Pharmacokinetic actions of exendin-4 in the rat: comparison with glucagon-like peptide-1. Drug Dev Res. 2001;53:260–7.
Stearns AT, Balakrishnan A, Tavakkolizadeh A. Impact of Roux-en-Y gastric bypass surgery on rat intestinal glucose transport. Am J Physiol Gastrointest Liver Physiol. 2009;297(5):G950–7.
Johannessen H, Kodama Y, Zhao CM, et al. Eating behavior and glucagon-like peptide-1-producing cells in interposed ileum and pancreatic islets in rats subjected to ileal interposition associated with sleeve gastrectomy. Obes Surg. 2013;23(1):39–49.
De Robertis E, Zito Marinosci G, Romano GM, et al. The use of sugammadex for bariatric surgery: analysis of recovery time from neuromuscular blockade and possible economic impact. Clinicoecon Outcomes Res. 2016;8:317–22.
Murphy R, Evennett NJ, Clarke MG, et al. Sleeve gastrectomy versus Roux-en-Y gastric bypass for type 2 diabetes and morbid obesity: double-blind randomised clinical trial protocol. BMJ Open. 2016;6(7):e011416.
Keidar A, Hershkop KJ, Marko L, et al. Roux-en-Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia. 2013;56(9):1914–8.
Mahdy T, Schou C. Efficacy of single anastomosis sleeve ileal (SASI) bypass for type-2 diabetic morbid obese patients: gastric bipartition, a novel metabolic surgery procedure: a retrospective cohort study. Int J Surg. 2016;34:28–34.
Salama TMS, Sabry K, El Ghamrini Y. Single anastomosis sleeve ileal bypass: new step in the evolution of bariatric surgeries. J Investig Surg. 2017;30(5):291–6.
Campos J, Ramos A, Szego T, et al. The role of metabolic surgery for patients with obesity grade I and clinically uncontrolled type 2 diabetes. Arq Bras Cir Dig. 2016;29:102–6.
Rizvi AA. The evolving role of bariatric surgery in patients with type 1 diabetes and obesity. Integr Obes Diabetes. 2016;2(2):195–9.
Vetter ML, Cardillo S, Rickels MR, et al. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150(2):94–103.
Ashrafian H, le Roux CW. Metabolic surgery and gut hormones—a review of bariatric entero-humoral modulation. Physiol Behav. 2009;97(5):620–31.
Jurowich CF, Otto C, Rikkala PR, et al. Ileal interposition in rats with experimental type 2 like diabetes improves glycemic control independently of glucose absorption. J Diabetes Res. 2015;2015:490365.
Nausheen S, Shah IH, Pezeshki A, et al. Effects of sleeve gastrectomy and ileal transposition, alone and in combination, on food intake, body weight, gut hormones, and glucose metabolism in rats. Am J Physiol Endocrinol Metab. 2013;305(4):E507–18.
Zhang H, Li J, Li Z, et al. Increased GLP-1 response after gavage-administration of glucose in UCP2-deficient mice. Horm Metab Res. 2012;44(2):86–90.
Imeryuz N, Yegen BC, Bozkurt A, et al. Glucagon-like peptide-1 inhibits gastric emptying via vagal afferent-mediated central mechanisms. Am J Phys. 1997;273(4 Pt 1):G920–7.
Zhao T, Parikh P, Bhashyam S, et al. Direct effects of glucagon-like peptide-1 on myocardial contractility and glucose uptake in normal and postischemic isolated rat hearts. J Pharmacol Exp Ther. 2006;317(3):1106–13.
Taqi E, Wallace LE, de Heuvel E, et al. The influence of nutrients, biliary-pancreatic secretions, and systemic trophic hormones on intestinal adaptation in a Roux-en-Y bypass model. J Pediatr Surg. 2010;45(5):987–95.
Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in Goto-kakizaki rats through an enhanced proglucagon gene expression and L-cell number. Surgery. 2007;142(1):74–85.
Li J, Lai D, Wu D. Laparoscopic Roux-en-Y gastric bypass versus laparoscopic sleeve gastrectomy to treat morbid obesity-related comorbidities: a systematic review and meta-analysis. Obes Surg. 2016;26(2):429–42.
Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–76.
Mahawar KK, Parikh C, Carr WR, et al. Primary banded Roux-en-Y gastric bypass: a systematic review. Obes Surg. 2014;24(10):1771–92.
Acknowledgments
The authors acknowledge Mr. Amirsalar Moazen Safaei and Mr. Aria Azarshahi for their help in proofreading this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding Resource
No fund received for this research.
Ethics Statements
This research commenced after fulfilling all of the ethical statements and receiving its approval from department of ethics in medical research. All procedures were conducted in agreement with the National Institutes of Health Guide for Care and Use of Laboratory Animals.
Rights and permissions
About this article
Cite this article
Keleidari, B., Mohammadi Mofrad, R., Shahabi Shahmiri, S. et al. The Impacts of Gastroileostomy Rat Model on Glucagon-like Peptide-1: a Promising Model to Control Type 2 Diabetes Mellitus. OBES SURG 28, 3246–3252 (2018). https://doi.org/10.1007/s11695-018-3312-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11695-018-3312-y