Abstract
Objective
To investigate whether Gli1 expression is important in relapse after radical operation of breast cancer.
Methods
Using immunohistochemistry, Gli1 expression was analyzed in human primary breast cancer (n=284) and paracancerous tissues (n=20), and also in local lymph nodes (n=28) and metastatic lymph nodes (n=28).
Results
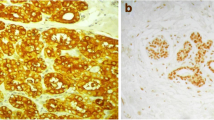
Initial analysis of Gli1 expression in a small cohort of 20 breast tumors and their paracancerous tissues showed a tendency towards Gli1 overexpression in breast cancer tissues (P<0.001). Further, Gli1 expression in 284 breast cancer tissue samples was analyzed and a significant correlation was found between increased expression of nuclear Gli1 and unfavorable recurrence-free survival (RFS) (P<0.05). The nuclear expression of Gli1 in metastatic lymph nodes following relapse after radical operation was much higher than that in the local lymph nodes of primary carcinoma (P<0.05). Most interestingly, the expression of Gli1 was much higher in the interstitial tissues of the relapsed group than of the non-relapsed group (P<0.001).
Conclusions
Breast cancer shows a high prevalence of Gli1 expression, which is significantly correlated with aggressive features and unfavorable RFS. Nuclear Gli1 overexpression, especially in the interstitial tissues, signified early relapse after radical operation of breast cancer.
Similar content being viewed by others
References
Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300.
Casey B, Hackett BP. Left-right axis malformations in man and mouse. Curr Opin Genet Dev 2000;10:257–261.
Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev 2001;15:3059–3087.
Schugar RC, Robbins PD, Deasy BM. Small molecules in stem cell self-renewal and differentiation. Gene Ther 2008;15:126–135.
Takezaki T, Hide T, Takanaga H, et al. Essential role of the Hedgehog signaling pathway in human gliomainitiating cells. Cancer Sci 2011;102:1306–1312.
Watkins DN, Berman DM, Burkholder SG, et al. Hedgehog signalling within airway epithelial progenitors and in small-cell lung cancer. Nature 2003;422:313–317.
Karhadkar SS, Bova GS, Abdallah N, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature 2004;431:707–712.
El-Zaatari M, Tobias A, Grabowska AM, et al. Deregulation of the sonic hedgehog pathway in the InsGas mouse model of gastric carcinogenesis. Br J Cancer 2007;96:1855–1861.
Cui W, Wang LH, Wen YY, et al. Expression and regulation mechanisms of Sonic Hedgehog in breast cancer. Cancer Sci 2010;101:927–933.
Souzaki M, Kubo M, Kai M, et al. Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer. Cancer Sci 2011;102:373–381.
Kasper M, Regl G, Frischauf AM, et al. GLI transcription factors: mediators of oncogenic Hedgehog signalling. Eur J Cancer 2006;42:437–445.
Borgquist S, Holm C, Stendahl M, et al. Oestrogen receptors alpha and beta show different associations to clinicopathological parameters and their co-expression might predict a better response to endocrine treatment in breast cancer. J Clin Pathol 2008;61:197–203.
ten Haaf A, Bektas N, von Serenyi S, et al. Expression of the glioma-associated oncogene homolog (GLI) 1 in human breast cancer is associated with unfavourable overall survival. BMC Cancer 2009;9:298.
Rydén L, Jirström K, Bendahl PO, et al. Tumor-specific expression of vascular endothelial growth factor receptor 2 but not vascular endothelial growth factor or human epidermal growth factor receptor 2 is associated with impaired response to adjuvant tamoxifen in premenopausal breast cancer. J Clin Oncol 2005;23:4695–4704.
Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989;63:181–187.
Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74–108.
Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature 1980;287:795–801.
Echelard Y, Epstein DJ, St-Jacques B, et al. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 1993;75:1417–1430.
Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell 1993;75:1431–1444.
Riddle RD, Johnson RL, Laufer E, et al. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 1993;75:1401–1416.
Chang DT, López A, von Kessler DP, et al. Products, genetic linkage and limb patterning activity of a murine hedgehog gene. Development 1994;120:3339–3353.
Hatsell S, Frost AR. Hedgehog signaling in mammary gland development and breast cancer. J Mammary Gland Biol Neoplasia 2007;12:163–173.
Bak M, Hansen C, Friis Henriksen K, et al. The human hedgehog-interacting protein gene: structure and chromosome map** to 4q31.21—>q31.3. Cytogenet Cell Genet 2001;92:300–303.
Rubin LL, de Sauvage FJ. Targeting the Hedgehog pathway in cancer. Nat Rev Drug Discov 2006;5:1026–1033.
Sasaki H, Nishizaki Y, Hui C, et al. Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 1999;126:3915–3924.
Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell 2004;6:103–115.
Buttitta L, Mo R, Hui CC, et al. Interplays of Gli2 and Gli3 and their requirement in mediating Shh-dependent sclerotome induction. Development 2003;130:6233–6243.
Dai P, Akimaru H, Tanaka Y, et al. Sonic Hedgehoginduced activation of the Gli1 promoter is mediated by GLI3. J Biol Chem 1999;274:8143–8152.
Ikram MS, Neill GW, Regl G, et al. GLI2 is expressed in normal human epidermis and BCC and induces GLI1 expression by binding to its promoter. J Invest Dermatol 2004;122:1503–1509.
Koyabu Y, Nakata K, Mizugishi K, et al. Physical and functional interactions between Zic and Gli proteins. J Biol Chem 2001;276:6889–6892.
Mao J, Maye P, Kogerman P, et al. Regulation of Gli1 transcriptional activity in the nucleus by Dyrk1. J Biol Chem 2002;277:35156–35161.
Kubo M, Nakamura M, Tasaki A, et al. Hedgehog signaling pathway is a new therapeutic target for patients with breast cancer. Cancer Res 2004;64:6071–6074.
Yanai K, Nagai S, Wada J, et al. Hedgehog signaling pathway is a possible therapeutic target for gastric cancer. J Surg Oncol 2007;95:55–62.
Kasai K, Inaguma S, Yoneyama A, et al. SCL/TAL1 interrupting locus derepresses GLI1 from the negative control of suppressor-of-fused in pancreatic cancer cell. Cancer Res 2008;68:7723–7729.
Stecca B, Ruiz i Altaba A. A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J 2009;28:663–676.
Rahnama F, Shimokawa T, Lauth M, et al. Inhibition of GLI1 gene activation by Patched1. Biochem J 2006;394:19–26.
Sotiriou C, Pusztai L. Gene-expression signatures in breast cancer. N Engl J Med 2009;360:790–800.
O’Toole SA, Machalek DA, Shearer RF, et al. Hedgehog overexpression is associated with stromal interactions and predicts for poor outcome in breast cancer. Cancer Res 2011;71:4002–4014.
Souzaki M, Kubo M, Kai M, et al. Hedgehog signaling pathway mediates the progression of non-invasive breast cancer to invasive breast cancer. Cancer Sci 2011;102:373–381.
Bernards R, Weinberg RA. A progression puzzle. Nature 2002;418:823.
Sanchez P, Hernández AM, Stecca B, et al. Inhibition of prostate cancer proliferation by interference with SONIC HEDGEHOG-GLI1 signaling. Proc Natl Acad Sci U S A 2004;101:12561–12566.
Stecca B, Mas C, Clement V, et al. Melanomas require HEDGEHOG-GLI signaling regulated by interactions between GLI1 and the RAS-MEK/AKT pathways. Proc Natl Acad Sci U S A 2007;104:5895–5900.
Feldmann G, Dhara S, Fendrich V, et al. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res 2007;67:2187–2196.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YH., Gao, HF., Wang, Y. et al. Overexpression of Gli1 in cancer interstitial tissues predicts early relapse after radical operation of breast cancer. Chin. J. Cancer Res. 24, 263–274 (2012). https://doi.org/10.1007/s11670-012-0263-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11670-012-0263-z