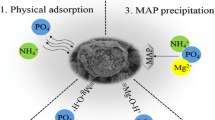
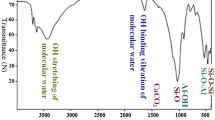
Abstract
This study reports the successful preparation of MgO nanomaterials through ultrasonic electrodeposition. The capability and mechanism of nanomaterials in removing Pb (II) from industrial soil under varying ultrasonic powers of 100, 150, and 200 W were investigated. Scanning electron microscopy (SEM), Transmission electron microscope (TEM), and x-ray diffractometry (XRD) were employed to analyze the surface morphology and phase composition of the nanomaterials. The adsorption capacity of MgO nanomaterials for Pb (II) was significantly enhanced at an ultrasonic power of 150 W, reaching a maximum adsorption capacity of 68.9 mg g−1. The adsorption process of Pb (II) onto the surface of MgO nanomaterials was analyzed using pseudo-second-order kinetic and Langmuir models. The formation of strong chemical bonds between Pb (II) and atoms on the surface of MgO nanomaterials resulted in the unique monolayer adsorption and chemisorption characteristics observed for Pb (II), as confirmed through Fourier transform infrared spectroscopy (FTIR) analysis. MgO nanomaterials revealed distinctive monolayer adsorption and chemisorption properties for the adsorption of Pb (II). The adsorption capacity of MgO nanomaterials for Pb (II) was significantly affected by temperature, with the highest adsorption amount observed at 30 °C. Moreover, the Langmuir model effectively described the adsorption behavior of MgO for Pb (II), displaying a correlation coefficient exceeding 0.99. The results reveal the influence of ultrasonic power on the adsorption capabilities of MgO nanomaterials prepared via ultrasonic electrodeposition, with optimal adsorption performance observed for MgO nanomaterials prepared at 150 W. Moreover, the MgO nanomaterials fabricated with 150 W ultrasonic power demonstrate good pH adaptability.











Similar content being viewed by others
References
B. Savun-Hekimoglu, Z. Isler, M. Hekimoglu, S. Burak, D. Karli, A. Yucekaya, E. Akpinar, and V.S. Ediger, Optimization of Wastewater Treatment Systems for Growing Industrial Parks, Sci. Total. Environ., 2023, 905, p 167223.
L.L. Li, Y.B. Shi, Y. Huang, A.L. **ng, and H. Xue, The Effect of Governance on Industrial Wastewater Pollution in China, Int. J. Environ. Res. Public Health, 2022, 19(15), p 9316.
G. Kanat and D. Yeter, Optimization and Reduction of Industrial Load in a Wastewater Basin, Desalin. Water Treat., 2020, 205, p 316–327.
Y.X. Tian, H. Zhang, S.C. Pan, Y.B. Yin, Z.Y. Jia, and H.F. Zhou, Amine-Functionalized Magnetic Microspheres from Lignosulfonate for Industrial Wastewater Purification, Int. J. Biol. Macromol., 2023, 224, p 133–142.
U. Kumari, H. Siddiqi, M. Bai, and B.C. Meikap, Calcium and Zirconium Modified Acid Activated Alumina for Adsorptive Removal of Fluoride: Performance Evaluation, Kinetics, Isotherm, Characterization and Industrial Wastewater Treatment, Adv. Power Technol., 2020, 31(5), p 2045–2060.
B.Y. Zhao, J.J. He, and L. Wang, Adsorption/Desorption Performance of Cellulose Membrane for Pb (ii), Green Process. Synth., 2023, 12(1), p 20230014.
Y.M. Desalegn, D.M. Andoshe, and T.D. Desissa, Composite of Bentonite/CoFe2O4/Hydroxyapatite for Adsorption of Pb (II), Mater. Res. Exp., 2021, 7(11), 115501.
P. Santoso, C. Anwar, D.S. Jumina, and K.O. Suharso, Adsorption Study of Pb (II) onto a Novel Calix[4]Resorcinarene-Chitosan Hybrid, Desalin Water Treatment, 2019, 143, p 268–273.
A. Alkhudhayri, F.A. Thagfan, S. Al-Quraishy, R. Abdel-Gaber, and M.A. Dkhil, Assessment of the Oxidative Status and Goblet Cell Response During Eimeriosis and After Treatment of Mice with Magnesium Oxide Nanoparticles, Saudi J. Biol. Sci., 2022, 29(2), p 1234–1238.
A. Deb, M. Kanmani, A. Debnath, K.L. Bhowmik and B. Saha, Ultrasonic Assisted Enhanced Adsorption of Methyl Orange Dye onto Polyaniline Impregnated Zinc Oxide Nanoparticles: Kinetic, Isotherm and Optimization of Process Parameters, Ultrason. Sonochem., 2019, 54, p 290–301.
B. Saha, S. Shaji, and A. Debnath, Fabrication of polyaniline based calcium ferrite nanocomposite and its application in sequestration of Victoria blue dye from wastewater, Journal of dispersion science and technology, 2023, 2273432.
Y.F. Guo, C. Tan, J. Sun, W.L. Li, J.B. Zhang, and C.W. Zhao, Biomass Ash Stabilized MgO Adsorbents for CO2 Capture Application, Fuel, 2020, 259, 116298.
J. Maruthai, K. Ramachandran, A. Muthukumarasamy, S. Chidabaram, M. Gaidi, and K. Daoudi, Bio Fabrication of 2D MgO/Ag Nanocomposite for Effective Environmental Utilization in Antibacterial, Anti-oxidant and Catalytic Applications, Surf. Interfaces, 2022, 30, 101921.
C. Ma, H. He, F. **a, Z. **ao, and Y. Liu, Performance of Ni-SiC Composites Deposited Using Magnetic-Field-Assisted Electrodeposition Under Different Magnetic-Field Directions, Ceram. Int., 2023, 49(22), p 35907–35916.
C. Ma, M. Jiang, W. Cui, and F. **a, Jet Pulse Electrodeposition and Characterization of Ni-AlN Nanocoatings in Presence of Ultrasound, Ceram. Int., 2018, 44(5), p 5163–5170.
F. **a, P. Yan, C. Ma, Y. Zhang, and H. Li, Pulse-Electrodeposited Ni/W-Al2O3 Nanocomposites at Different Current Densities, J. Nanopart. Res., 2023, 25, p 208.
C. Ma, D. Zhao, F. **a, H. **a, T. Williams, and H. **ng, Ultrasonic-Assisted Electrodeposition of Ni-Al2O3 Nanocomposites at Various Ultrasonic Powers, Ceram. Int., 2020, 46(5), p 6115–6123.
J.K. Liu, L.J. Yang, Z.L. Song, and C. Xu, Microstructures and Capacitance Performance of MnO2 Films Fabricated by Ultrasonic-assisted Electrodeposition, Appl. Surf. Sci., 2019, 478, p 94–102.
M.R. Akbarpour and F.G. Asl, Fabrication of High-Performance Graphene/Nickel-Cobalt Composite Coatings using Ultrasonic-Assisted Pulse Electrodeposition, Ceram. Int., 2023, 49(9), p 13829–13835.
M.S. Rajput, P.M. Pandey, and S. Jha, Fabrication of Nano-Sized Grain Micro Features Using ultrasOnic-Assisted Jet Electrodeposition with Pulsed Current Supply, Proc. Inst. Mech. Eng. Part B-J. Eng. Manuf., 2014, 11(228), p 1338–1349.
M. Wu, W. Jia, and P. Lv, Electrodepositing Ni-TiN Nanocomposite Layers with Applying Action of Ultrasonic Waves, Proc. Eng.., 2017, 174, p 717–723.
F. **a, P. Yan, C. Ma, B. Wang, and Y. Liu, Effect of Different Heat-Treated Temperatures upon Structural and Abrasive Performance of Ni-TiN Composite Nanocoatings, J. Mater. Res. Technol., 2023, 27, p 2874–2881.
T. Liu, C. Li, Q. Li, L. Li, F. **a, H. **ng, and C. Ma, Synthesis and Wear Characterization of Ultrasonic Electrodeposited Ni-TiN Thin Coatings, Int. J. Electrochem. Sci., 2021, 16(3), 151028.
C. Ma, D. Zhao, and Z. Ma, Effects of Duty Cycle and Pulse Frequency on Microstructures and Properties of Electrodeposited Ni-Co-SiC Nanocoatings, Ceram. Int., 2020, 46(8), p 12128–12137.
X.L. Li, G. Tang, D. Zhang, L.J. Wu, S.J. Lu, Y.Z. Zhang, X. Cao, W. Cheng, J.T. Feng, W. Yan, B.Z. Pan, L. Li, Z.B. Li, and X. Zheng, Fouling Control in Ultrafiltration of Secondary Effluent Using Polyaniline/TiO2 Adsorption and Subsequent Treatment of Desorption Eluate Using Electrochemical Oxidation, Chem. Eng. J., 2020, 382, 122915.
L. Tognotti, M. Flytzani-Stephanopoulos, A.F. Sarofim, H. Kopsinis, and M. Stoukides, Study of Adsorption-Desorption of Contaminants on Single Soil Particles Using the Electrodynamic Thermogravimetric Analyzer, Environ. Ence Technol., 1991, 25(1), p 104–109.
H.R. Mahmoud, S.A. El-Molla, and M.A. Naghmash, Novel Mesoporous MnO2/SnO2 Nanomaterials Synthesized by Ultrasonic-Assisted Co-precipitation Method and Their Application in the Catalytic Decomposition of Hydrogen Peroxide, Ultrasonics, 2019, 95, p 95–103.
H. Doweidar, G. El-Damrawi, E. Mansour, and R.E. Fetouh, Structural Role of MgO and PbO in MgO-PbO-B2O3 Glasses as Revealed by FTIR; a New Approach, J. Non-Cryst. Solids, 2012, 358(5), p 941–946.
P. Das, A. Debnath, and B. Saha, Ultrasound-Assisted Enhanced and Rapid Uptake of Anionic Dyes from the Binary System onto MnFe2O4/Polyaniline Nanocomposite at Neutral pH, Appl. Organomet. Chem., 2020, 34(8), e5711.
P. Das, S. Nisa, A. Debnath, and B. Saha, Enhanced Adsorptive Removal of Toxic Anionic Dye by Novel Magnetic Polymeric Nanocomposite: Optimization of Process Parameters, J. Dispersion Sci. Technol., 2022, 43(6), p 880–895.
T. Mahmood, M.T. Saddique, A. Naeem, S. Mustafa, N. Zeb, K.H. Shah, and M. Waseem, Kinetic and Thermodynamic study of Cd (II), Co (II) and Zn (II) Adsorption from Aqueous Solution by NiO, Chem. Eng. J., 2011, 171(3), p 935–940.
D. Ursueguia, E. Diaz, and S. Ordonez, Adsorption of Methane and Nitrogen on Basolite MOFs: Equilibrium and Kinetic Studies, Microporous Mesoporous Mater., 2020, 298, 110048.
S.K. Sahoo, G.K. Panigrahi, A. Arzoo, A. Sahoo, A.K. Pradhan, M.K. Sahu, J.K. Sahoo, and A. Dalbehera, Biological Synthesis of GO-MgO Nanomaterial using Azadirachta Indica Leaf Extract: A Potential Bio-adsorbent for Removing Cr (VI) ions from Aqueous Media, Biochem. Eng. J., 2022, 177, p 108272.
I.W. Sutapa, A.W. Wahab, P. Taba, and N.L. Nafie, Synthesis and Structural Analysis of Magnesium Oxide Nanomaterial Using Ethanol as Polymerization Solvent, Indonesian J. Fund. Appl. Chem., 2019, 4(2), p 82–90.
Z. Su, S.Y. Ning, Z.Y. Li, and S.C. Zhang, High-Efficiency Separation of Palladium from Nitric Acid Solution Using a Silica-Polymer-Based Adsorbent isoPentyl-BTBP/SiO2-P, J. Environ. Chem. Eng., 2022, 10(3), 107928.
A. Deb, A. Debnath, and B. Saha, Sono-Assisted Enhanced Adsorption of Eriochrome Black-T Dye onto a Novel Polymeric Nanocomposite: Kinetic, Isotherm, and Response Surface Methodology Optimization, J. Dispers. Technol., 2020, 42(11), p 1579–1592.
A. Deb, S. Das, and A. Debnath, Fabrication and Characterization of Organometallic Nanocomposite for Efficient Abatement of Dye Laden Wastewater: CCD Optimization, Adsorption Mechanism, Co-existing Ions, and Cost Analysis, Chem. Phys. Lett., 2023, 830, p 140820.
Acknowledgments
This paper was supported by the Liaoning Provincial Engineering Research Center for High-Value Utilization of Magnesite (Granted nos. LMNKY202310, LMNKZ202303 and LMNKY202309), Basic scientific research project of Liaoning Provincial Department of Education (Granted no. JYTMS20230067), General project of Education Department of Liaoning Province (Granted no. LJKZ1196), and Intercollegiate Cooperation (Collaborative Innovation) project of ordinary undergraduate colleges and universities in Liaoning Province (Granted no. L202161).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Zhao, Z., He, L. et al. Adsorption Capacity and Mechanism of MgO Nanomaterials Prepared by Ultrasonic Electrodeposition for Pb (II). J. of Materi Eng and Perform (2024). https://doi.org/10.1007/s11665-024-09556-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11665-024-09556-7