Abstract
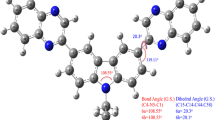
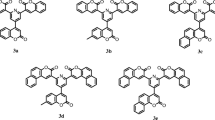
Triphenylamine derived bis- and tris-branched donor-pi-acceptor coumarins with acetyl and benzothiazolyl acceptors are studied for their linear and nonlinear optical properties that originate from their photophysical and molecular structure. Plots of solvent polarities versus the Stokes shift, frontier molecular orbital analysis and Generalised Mulliken Hush analysis have established their strong charge transfer character supported by the strong emission solvatochromism of these chromophores. On the basis of excited state intramolecular charge transfer, the first-, second- and third-order polarizability of these dyes are determined by a solvatochromic method and supported by density functional theory calculations using CAM-B3LYP/6-31g(d). Compared to the acetyl group, the benzothiazolyl group is a strong acceptor, and its corresponding derivatives show enhanced absorption, emission maxima and non-linear optical response. Bond length alternation and bond order alternation analysis reveals that these chromophores approach the cyanine-like framework which is responsible for maximum perturbation to produce high nonlinear optical response. Third order nonlinear susceptibility for dyes 1 and 2 is determined by Z-scan measurement. All of these methods are used to determine the nonlinear optical properties, and thermogravimetric analysis suggests that these chromophores are thermally robust and efficient nonlinear optical materials.
Similar content being viewed by others
References
L.W. Duarte and F.J. Hillman, Dye Laser Principles, with Applications (San Diego: Academic Press Inc., 1990).
B. Liu, R. Wang, W. Mi, X. Li, and H. Yu, J. Mater. Chem. 22, 15379 (2012).
S.K. Lanke and N. Sekar, J. Fluoresc. 26, 949 (2016).
P.R. Varanasi, A.K.-Y. Jen, J. Chandrasekhar, I.N.N. Namboothiri, and A. Rathna, J. Am. Chem. Soc. 118, 12443 (1996).
I. Baraldi, E. Benassi, S. Ciorba, M. Šindler-Kulyk, I. Škorić, and A. Spalletti, Chem. Phys. 361, 61 (2009).
C.G. Fortuna, U. Mazzucato, G. Musumarra, D. Pannacci, and A. Spalletti, J. Photochem. Photobiol. A Chem. 216, 66 (2010).
B. Carlotti, A. Spalletti, M. Sindler-Kulyk, and F. Elisei, Phys. Chem. Chem. Phys. 13, 4519 (2011).
Y.V. Pereverzev, K.N. Gunnerson, O.V. Prezhdo, P.A. Sullivan, Y. Liao, B.C. Olbricht, A.J.P. Akelaitis, A.K.-Y. Jen, and L.R. Dalton, J. Phys. Chem. C 112, 4355 (2008).
L.R. Dalton, P.A. Sullivan, and D.H. Bale, Chem. Rev. 110, 25 (2010).
X. Liu, J.M. Cole, P.G. Waddell, T.-C. Lin, J. Radia, and A. Zeidler, J. Phys. Chem. A 116, 727 (2012).
J. Qi, W. Qiao, and Z.Y. Wang, Chem. Rec. 16, 1531 (2016).
R.L. Gieseking, C. Risko, and J.-L. Brédas, J. Phys. Chem. Lett. 6, 2158 (2015).
T.G. Pavlopoulos, IEEE J. Quantum Electron. 9, 510 (1973).
X. Tang, W. Liu, J. Wu, C.-S. Lee, J. You, and P. Wang, J. Org. Chem. 75, 7273 (2010).
S. Roquet, A. Cravino, P. Leriche, O. Alévêque, P. Frère, and J. Roncali, J. Am. Chem. Soc. 128, 3459 (2006).
P. Leriche, P. Frère, A. Cravino, O. Alévêque, and J. Roncali, J. Org. Chem. 72, 8332 (2007).
M. He, R.J. Twieg, U. Gubler, D. Wright, and W.E. Moerner, Chem. Mater. 15, 1156 (2003).
C.N. LaFratta, J.T. Fourkas, T. Baldacchini, and R.A. Farrer, Angew. Chemie Int. Ed. 46, 6238 (2007).
M. Behl, E. Hattemer, M. Brehmer, and R. Zentel, Macromol. Chem. Phys. 203, 503 (2002).
J.L. Hua, B. Li, F.S. Meng, F. Ding, S.X. Qian, and H. Tian, Polymer (Guildf) 45, 7143 (2004).
C. Allain, F. Schmidt, R. Lartia, G. Bordeau, C. Fiorini-Debuisschert, F. Charra, P. Tauc, and M.-P. Teulade-Fichou, ChemBioChem 8, 424 (2007).
G.S. He, L.-S. Tan, Q. Zheng, and P.N. Prasad, Chem. Rev. 108, 1245 (2008).
Y. Yu, Y. Cui, Y. Yang, and G. Qian, RSC Adv. 6, 81969 (2016).
C. Hu, F. Liu, H. Zhang, F. Huo, Y. Yang, H. Wang, H. **ao, Z. Chen, J. Liu, L. Qiu, Z. Zhen, X. Liu, and S. Bo, J. Mater. Chem. C 3, 11595 (2015).
W. Chen, Z.-R. Li, D. Wu, F.-L. Gu, X.-Y. Hao, B.-Q. Wang, R.-J. Li, and C.-C. Sun, J. Chem. Phys. 121, 10489 (2004).
Y. Li, Z.-R. Li, D. Wu, R.-Y. Li, X.-Y. Hao, and C.-C. Sun, J. Phys. Chem. B 108, 3145 (2004).
N. Mataga, Y. Kaifu, and M. Koizumi, Bull. Chem. Soc. Jpn. 29, 465 (1956).
Z.R. Grabowski, K. Rotkiewicz, and W. Rettig, Chem. Rev. 103, 3899 (2003).
B. Valeur, in Molecular Fluorescence (New York: Wiley, 2001), pp. 200–225.
J.L. Oudar and D.S. Chemla, J. Chem. Phys. 66, 2664 (1977).
B. Carlotti, R. Flamini, I. Kikaš, U. Mazzucato, and A. Spalletti, Chem. Phys. 407, 9 (2012).
L. Chen, Y. Cui, X. Mei, G. Qian, and M. Wang, Dyes Pigments 72, 293 (2007).
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, G.A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B.G. Janesko, R. Gomperts, B. Mennucci, H.P. Hratchian, J.V. Ortiz, A.F. Izmaylov, J.L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V.G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J.A. Montgomery, Jr., J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, J.M. Millam, M. Klene, C. Adamo, R. Cammi, J.W. Ochterski, R.L. Martin, K. Morokuma, O. Farkas, J.B. Foresman, and D.J. Fox, Gaussian 09, Revision A.02 (Wallingford, CT: Gaussian, Inc., 2016).
R. Dennington, T.A. Keith, and J.M. Millam, GaussView, Version 5 (Semichem Inc., 2009).
W. Kohn and L.J. Sham, Phys. Rev. 140, A1133 (1965).
R. Menzel, D. Ogermann, S. Kupfer, D. Weiß, H. Görls, K. Kleinermanns, L. González, and R. Beckert, Dyes Pigments 94, 512 (2012).
C. Lee, W. Yang, and R.G. Parr, Phys. Rev. B 37, 785 (1988).
M.W. Wong, M.J. Frisch, and K.B. Wiberg, J. Am. Chem. Soc. 113, 4776 (1991).
L.E. Johnson, L.R. Dalton, and B.H. Robinson, Acc. Chem. Res. 47, 3258 (2014).
O.D. Fominykh, A.V. Sharipova, and MYu Balakina, Int. J. Quantum Chem. 116, 103 (2016).
J. Tomasi, B. Mennucci, and R. Cammi, Chem. Rev. 105, 2999 (2005).
S. Kothavale and N. Sekar, Dye. Pigment. 136, 116 (2017).
N.J. Turro, Modern Molecular Photochemistry (Menlo Park, CA: Benjamin/Cummings Publishing Co., Inc., 1978).
B.J. Coe, J.A. Harris, I. Asselberghs, K. Clays, G. Olbrechts, A. Persoons, J.T. Hupp, R.C. Johnson, S.J. Coles, M.B. Hursthouse, and K. Nakatani, Adv. Funct. Mater. 12, 110 (2002).
E. Lippert, Zeitschrift Für Elektrochemie, Berichte Der Bunsengesellschaft Für Phys. Chemie 61, 962 (1957).
I.D.L. Albert, T.J. Marks, and M.A. Ratner, J. Am. Chem. Soc. 120, 11174 (1998).
B.J. Coe, J. Fielden, S.P. Foxon, J.A. Harris, M. Helliwell, B.S. Brunschwig, I. Asselberghs, K. Clays, J. Garín, and J. Orduna, J. Am. Chem. Soc. 132, 10498 (2010).
Shawn M. Abernathy and Robert R. SharpView, J. Chem. Phys. 106, 9032 (1997).
C. Creutz, M.D. Newton, and N. Sutin, J. Photochem. Photobiol. A Chem. 82, 47 (1994).
M. Rust, J. Lappe, and R.J. Cave, J. Phys. Chem. A 106, 3930 (2002).
M.D. Newton, J. Electroanal. Chem. 438, 3 (1997).
A. Akella, S.L. Sochava, and L. Hesselink, Opt. Lett. 22, 919 (1997).
M. Matsui, M. Suzuki, M. Hayashi, K. Funabiki, Y. Ishigure, Y. Doke, and H. Shiozaki, Bull. Chem. Soc. Jpn 76, 607 (2003).
C.R. Moylan, R.J. Twieg, V.Y. Lee, S.A. Swanson, K.M. Betterton, and R.D. Miller, J. Am. Chem. Soc. 115, 12599 (1993).
R.J. Jeng, Y.M. Chen, A.K. Jain, J. Kumar, and S.K. Tripathy, Chem. Mater. 4, 972 (1992).
L.A. Padilha, S. Webster, H. Hu, O.V. Przhonska, D.J. Hagan, E.W. Van Stryland, M.V. Bondar, I.G. Davydenko, Y.L. Slominsky, and A.D. Kachkovski, Chem. Phys. 352, 97 (2008).
J. Mattu, T. Johansson, and G.W. Leach, J. Phys. Chem. C 111, 6868 (2007).
Y. Erande, M.C. Sreenath, S. Chitrambalam, I.H. Joe, and N. Sekar, Opt. Mater. (Amst.) 66, 494 (2017).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Erande, Y., Kothavale, S., Sreenath, .C. et al. Triphenylamine Derived 3-Acetyl and 3-Benzothiazolyl Bis and Tris Coumarins: Synthesis, Photophysical and DFT Assisted Hyperpolarizability Study. J. Electron. Mater. 47, 1431–1446 (2018). https://doi.org/10.1007/s11664-017-5925-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11664-017-5925-7