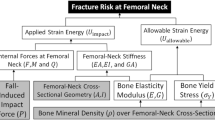
Abstract
Summary
Trends toward more favorable improvement of the cortical bone parameters by once-weekly (56.5 μg once a week) and twice-weekly teriparatide (28.2 μg twice a week), and that of the trabecular bone parameters by once-daily (1/D) teriparatide (20 μg/day once a day) were shown.
Purpose
To examine the effects of differences in the amount of teriparatide (TPTD) per administration and its dosing frequency on the bone structure in the proximal femur by dual-energy X-ray absorptiometry (DXA)-based 3D-modeling (3D-SHAPER software).
Methods
This was a multicenter retrospective study. Patients aged 50 years or older with primary osteoporosis who continuously received once-/twice-weekly (1・2/W, n = 60) or 1/D TPTD (n = 14) administration for at least one year were included in the study. Measurement regions included the femoral neck (FN), trochanter (TR), femoral shaft (FS), and total proximal hip (TH). Concurrently, the bone mineral density (BMD) and Trabecular Bone Score (TBS) were measured.
Results
The cross-sectional area, cross-sectional moment of inertia, and section modulus in the FS were significantly improved in the 1・2/W TPTD group, as compared to the 1/D TPTD group. However, significant improvement of the cortical thickness and buckling ratio in the FN was observed in the 1/D TPTD group, as compared to the 1・2/W TPTD group. Trabecular BMD values in the FS and TH were significantly increased in the 1/D TPTD group, as compared to the 1・2/W TPTD group, while the cortical BMD values in the TR, FS, and TH were significantly increased in the 1・2/W TPTD group, as compared to the 1/D TPTD group.
Conclusion
Trends toward more favorable improvement of the cortical bone by 1・2/W TPTD and that of the trabecular bones by 1/D TPTD were observed.





Similar content being viewed by others
Data Availability Statement
The biochemical data used to support the findings of this study belong to the study team. For confidentiality reasons, the datasets are not publicly available. However, the datasets can be obtained from the corresponding author with permission from the study team upon reasonable request.
References
Orimo H, Nakamura T, Hosoi T et al (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos 7:3–20. https://doi.org/10.1007/s11657-012-0109-9
Takusari E, Sakata K, Hashimoto T et al (2020) Trends in hip fracture incidence in Japan: estimates based on nationwide hip fracture surveys from 1992 to 2017. JBMR Plus 5:e10428. https://doi.org/10.1002/jbm4.10428
Saag KG, Petersen J, Brandi ML et al (2017) Romosozumab or Alendronate for Fracture Prevention in Women with Osteoporosis. N Engl J Med 377:1417–1427. https://doi.org/10.1056/NEJMoa1708322
Al-Lamee R, Thompson D, Dehbi HM et al (2018) Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 391:31–40. https://doi.org/10.1016/S0140-6736(17)32714-9
Hagino H, Sugimoto T, Tanaka S et al (2021) A randomized, controlled trial of 1/W teriparatide injection versus alendronate in patients at high risk of osteoporotic fracture: primary results of the Japanese Osteoporosis Intervention Trial-05. Osteoporos Int 32:2301–2311. https://doi.org/10.1007/s00198-021-05996-2
Nakamura T, Sugimoto T, Nakano T et al (2012) Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] 1/W Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab 97:3097–3106. https://doi.org/10.1210/jc.2011-3479
Sugimoto T, Shiraki M, Fukunaga M et al (2019) Study of 2/W injections of Teriparatide by comparing efficacy with 1/W injections in osteoporosis patients: the TWICE study. Osteoporos Int 30:2321–2331. https://doi.org/10.1007/s00198-019-05111-6
Tsujimoto M, Chen P, Miyauchi A et al (2011) PINP as an aid for monitoring patients treated with teriparatide. Bone 48:798–803. https://doi.org/10.1016/j.bone.2010.12.006
Miyauchi A, Matsumoto T, Sugimoto T et al (2010) Effects of teriparatide on bone mineral density and bone turnover markers in Japanese subjects with osteoporosis at high risk of fracture in a 24-month clinical study: 12-month, randomized, placebo-controlled, double-blind and 12-month open-label phases. Bone 47:493–502. https://doi.org/10.1016/j.bone.2010.05.022
Yamane H, Takakura A, Shimadzu Y et al (2017) Acute development of cortical porosity and endosteal naïve bone formation from the daily but not weekly short-term administration of PTH in rabbit. PLoS One 12:e0175329. https://doi.org/10.1371/journal.pone.0175329
Chiba K, Okazaki N, Kurogi A et al (2022) Randomized controlled trial of daily teriparatide, weekly high-dose teriparatide, or bisphosphonate in patients with postmenopausal osteoporosis: The TERABIT study. Bone 160:116416. https://doi.org/10.1016/j.bone.2022.116416
Soen S, Fukunaga M, Sugimoto T et al (2013) Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab 31:247–257. https://doi.org/10.1007/s00774-013-0447-8
Takada J, Beck TJ, Iba K et al (2007) Structural trends in the aging proximal femur in Japanese postmenopausal women. Bone 41:97–102. https://doi.org/10.1016/j.bone.2007.04.178
Shuhart CR, Yeap SS, Anderson PA et al (2019) Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom 22:453–471. https://doi.org/10.1016/j.jocd.2019.07.001
Humbert L, Martelli Y, Fonolla R et al (2017) 3D-DXA: Assessing the Femoral Shape, the Trabecular Macrostructure and the Cortex in 3D from DXA images. IEEE Trans Med Imaging 36:27–39. https://doi.org/10.1109/TMI.2016.2593346
Humbert L, Hazrati Marangalou J et al (2016) Technical note: cortical thickness and density estimation from clinical CT using a prior thickness-density relationship. Med Phys 43:1945–1954. https://doi.org/10.1118/1.4944501
Clotet J, Martelli Y, Di Gregorio S et al (2018) Structural parameters of the Proximal Femur by 3-Dimensional Dual-Energy X-ray absorptiometry software: comparison with quantitative computed tomography. J Clin Densitom 21:550–562. https://doi.org/10.1016/j.jocd.2017.05.002
Iki M, Winzenrieth R, Tamaki J et al (2021) Predictive ability of novel volumetric and geometric indices derived from dual-energy X-ray absorptiometric images of the proximal femur for hip fracture compared with conventional areal bone mineral density: the Japanese Population-based Osteoporosis (JPOS) Cohort Study. Osteoporos Int 32:2289–2299. https://doi.org/10.1007/s00198-021-06013-2
Sone T, Humbert L, Lopez M, Winzenrieth R (2021) Assessment of femoral shape, trabecular and cortical bone in Japanese subjects using DXA-based 3D modelling. Paper presented at the American Society for Bone and Mineral Research 2021 Annual Meeting, Toronto, Canada, October 1-4, 2021
Zullo AR, Lee Y, Lary C et al (2021) Comparative effectiveness of denosumab, teriparatide, and zoledronic acid among frail older adults: a retrospective cohort study. Osteoporos Int 32:565–573. https://doi.org/10.1007/s00198-020-05732-2
Tsukamoto M, Okimoto N, Mori M et al (2022) Bone microstructure changes due to once-/twice-weekly teriparatide administration: A report of five cases using high-resolution peripheral quantitative computed tomography. Modern Rheumatol Case Rep 6:301–304. https://doi.org/10.1093/mrcr/rxab048
Winzenrieth R, Humbert L, Di Gregorio S et al (2018) Effects of osteoporosis drug treatments on cortical and trabecular bone in the femur using DXA-based 3D modeling. Osteoporos Int 29:2323–2333. https://doi.org/10.1007/s00198-018-4624-4
Winzenrieth R, Ominsky MS, Wang Y et al (2021) Differential effects of abaloparatide and teriparatide on hip cortical volumetric BMD by DXA-based 3D modeling. Osteoporos Int 32:575–583. https://doi.org/10.1007/s00198-020-05806-1
Ettinger B, San Martin J, Crans G et al (2004) Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res 19:745–751. https://doi.org/10.1359/JBMR.040117
Miller PD, Delmas PD, Lindsay R et al (2008) Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab 93:3785–3793. https://doi.org/10.1210/jc.2008-0353
Neer RM, Arnaud CD, Zanchetta JR et al (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441. https://doi.org/10.1056/NEJM200105103441904
Acknowledgements
Author and co-authors are indebted to Kiichi Nonaka (TOYO MEDIC CO., LTD.; Tokyo Japan), who provided technical support and analyzed the data, and Keishi Mori (Shido Inc.).
Funding
This study was conducted with financial support from Asahi-Kasei Pharmaceutical Co., Ltd. (Tokyo, Japan).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards and with the protocol approved by the ethics review committee of Sapporo Maruyama Orthopedic Hospital (approved on October 22, 2021; approval No. 000042). Since it was a retrospective study, for this type of study formal consent is not required. Therefore, the opt-out procedure was used.
Conflict of interest
Junichi Takada received fees for lectures and medical advice from Asahi-Kasei Pharmaceutical Co., Ltd. Nobukazu Okimoto received consulting fees from Asahi-Kasei Pharmaceutical Co., Ltd. And Tei** Pharma Ltd. and received payments for lectures, including speakers’ bureau fees, from Asahi-Kasei Pharmaceutical Co., Ltd., Amgen K. K., Chugai Pharmaceutical Co., Daiichi-Sankyo Co. Ltd., Towa Pharmaceutical Co., Ltd., and Tei** Pharma Ltd. Manabu Tsukamoto received payments for lectures, including speakers’ bureau fees, from Asahi-Kasei Pharmaceutical Co., Ltd. Kousuke Iba holds an endowed chair at the Department of Musculoskeletal Anti-aging Medicine, Sapporo Medical University. Satoshi Ikeda received fees for medical advice from Asahi-Kasei Pharmaceutical Co., Ltd. and fees for lectures from Asahi-Kasei Pharmaceutical Co., Ltd. and Daiichi-Sankyo Co. Ltd.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takada, J., Okimoto, N., Tsukamoto, M. et al. Effects of differences in dose and frequency of teriparatide on bone structure in Proximal Femur. – Analysis by DXA-based 3D-modeling (3D-SHAPER Software) –TRIPLE-BONE study (The effects of TeRIParatide preparation on bone mineraL density increase and BONE structure). Arch Osteoporos 19, 55 (2024). https://doi.org/10.1007/s11657-024-01415-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11657-024-01415-1