Abstract
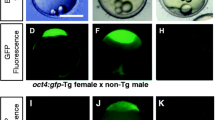
The establishment of induced pluripotent stem (iPS) cell technology in fish could facilitate the establishment of novel cryopreservation techniques for storing selected aquaculture strains as frozen cells. In order to apply iPS cell technology to fish, we established a transgenic zebrafish line, Tg(Tru.oct4:EGFP), using green fluorescent protein (GFP) expression under the control of the oct4 gene promoter as a marker to evaluate multipotency in iPS cell preparations. We used the oct4 promoter from fugu (Takifugu rubripes) due to the compact nature of the fugu genome and to facilitate future applications of this technology in marine fishes. During embryogenesis, maternal GFP fluorescence was observed at the cleavage stage and zygotic GFP expression was observed from the start of the shield stage until approximately 24 h after fertilization. gfp messenger RNA (mRNA) was expressed by whole embryonic cells at the shield stage, and then restricted to the caudal neural tube in the latter stages of embryogenesis. These observations showed that GFP fluorescence and the regulation of gfp mRNA expression by the exogenous fugu oct4 promoter are well suited for monitoring endogenous oct4 mRNA expression in embryos. Bisulfite sequencing revealed that the rate of CpG methylation in the transgenic oct4 promoter was high in adult cells (98%) and low in embryonic cells (37%). These findings suggest that, as with the endogenous oct4 promoter, demethylation and methylation both take place normally in the transgenic oct4 promoter during embryogenesis. The embryonic cells harvested at the shield stage formed embryonic body-like cellular aggregates and maintained GFP fluorescence for 6 d when cultured on Transwell-COL Permeable Supports or a feeder layer of adult fin cells. Loss of GFP fluorescence by cultured cells was correlated with cellular differentiation. We consider that the Tg(Tru.oct4:EGFP) zebrafish line established here is well suited for monitoring multipotency in multipotent zebrafish cell cultures and for iPS cell preparation.




Similar content being viewed by others
References
Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, Fahrenkrug SC, Carlson DF, Voytas DF, Clark KJ, Essner JJ, Ekker SC (2012) In vivo genome editing using a high-efficiency TALEN system. Nature 491:114–118
Boland MJ, Hazen JL, Nazor KL, Rodriguez AR, Gifford W, Martin G, Kupriyanov S, Baldwin KK (2009) Adult mice generated from induced pluripotent stem cells. Nature 461:91–94
Burgess S, Reim G, Chen W, Hopkins N, Brand M (2002) The zebrafish spiel-ohne-grenzen (spg) gene encodes the POU domain protein Pou2 related to mammalian Oct4 and is essential for formation of the midbrain and hindbrain, and for pre-gastrula morphogenesis. Development 129:905–916
Chambers I (2004) The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells 6:386–391
Collodi P, Kamei Y, Ernst T, Miranda C, Buhler DR, Barnes DW (1992) Culture of cells from zebrafish (Brachydanio rerio) embryo and adult tissues. Cell Biol Toxicol 8:43–61
Doetschman T, Gregg RG, Maeda N, Hooper ML, Melton DW, Thompson S, Smithies O (1987) Targetted correction of a mutant HPRT gene in mouse embryonic stem cells. Nature 330:576–578
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Fan L, Collodi P (2006) Zebrafish embryonic stem cells. Methods Enzymol 418:64–77
Fan L, Crodian J, Liu X, Alestrom A, Alestrom P, Collodi P (2004) Zebrafish embryo cells remain pluripotent and germ-line competent for multiple passages in culture. Zebrafish 1:21–26
Hong Y, Schartl M (2006) Isolation and differentiation of medaka embryonic stem cells. Methods Mol Biol 329:3–16
Hong Y, Winkler C, Schartl M (1996) Pluripotency and differentiation of embryonic stem cell lines from the medakafish (Oryzias latipes). Mech Dev 60:33–44
Hong Y, Winkler C, Liu T, Chai G, Schartl M (2004) Activation of the mouse Oct4 promoter in medaka embryonic stem cells and its use for ablation of spontaneous differentiation. Mech Dev 121:933–943
Hwang WY, Fu Y, Reyon D, Maeder ML, Tsai SQ, Sander JD, Peterson RT, Yeh JR, Joung JK (2013) Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat Biotechnol 31:227–229
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF (1995) Stages of embryonic development of the zebrafish. Dev Dyn 203:253–310
Lindeman LC, Winata CL, Aanes H, Mathavan S, Alestrom P, Collas P (2010) Chromatin states of developmentally-regulated genes revealed by DNA and histone methylation patterns in zebrafish embryos. Int J Dev Biol 54:803–813
Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95:379–391
Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Scholer HR, Suda T (2003) Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol 258:209–225
Parvin MS, Okuyama N, Inoue F, Islam ME, Kawakami A, Takeda H, Yamasu K (2008) Autoregulatory loop and retinoic acid repression regulate pou2/pou5f1 gene expression in the zebrafish embryonic brain. Dev Dyn 237:1373–1388
Robinton DA, Daley GQ (2012) The promise of induced pluripotent stem cells in research and therapy. Nature 481:295–305
Rossello RA, Chen CC, Dai R, Howard JT, Hochgeschwender U, Jarvis ED (2013) Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. Elife 2:e00036
Schwartzberg PL, Goff SP, Robertson EJ (1989) Germ-line transmission of a c-abl mutation produced by targeted gene disruption in ES cells. Science 246:799–803
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
Thermes V, Grabher C, Ristoratore F, Bourrat F, Choulika A, Wittbrodt J, Joly JS (2002) I-SceI meganuclease mediates highly efficient transgenesis in fish. Mech Dev 118:91–98
Thomas KR, Capecchi MR (1987) Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51:503–512
Wakamatsu Y, Ozato K, Sasado T (1994) Establishment of a pluripotent cell line derived from a medaka (Oryzias latipes) blastula embryo. Mol Mar Biol Biotechnol 3:185–191
Yoshimizu T, Sugiyama N, De Felice M, Yeom YI, Ohbo K, Masuko K, Obinata M, Abe K, Scholer HR, Matsui Y (1999) Germline-specific expression of the Oct-4/green fluorescent protein (GFP) transgene in mice. Dev Growth Differ 41:675–684
Zijlstra M, Li E, Sajjadi F, Subramani S, Jaenisch R (1989) Germ-line transmission of a disrupted β 2microglobulin gene produced by homologous recombination in embryonic stem cells. Nature 342:435–438
Acknowledgments
This research was supported by Grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan to TS (Chosenteki-Hoga: No. 22658063) and by The Towa Foundation for Food Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Hiroyuki Kato and Kota Abe contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kato, H., Abe, K., Yokota, S. et al. Establishment of oct4:gfp transgenic zebrafish line for monitoring cellular multipotency by GFP fluorescence. In Vitro Cell.Dev.Biol.-Animal 51, 42–49 (2015). https://doi.org/10.1007/s11626-014-9805-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-014-9805-7