Abstract
Purpose
To investigate the role of magnetic resonance imaging (MRI) based on radiomics using T2-weighted imaging fat suppression (T2WI-FS) and contrast enhanced T1-weighted imaging (CE-T1WI) sequences in differentiating T1-category nasopharyngeal carcinoma (NPC) from nasopharyngeal lymphoid hyperplasia (NPH).
Materials and methods
This study enrolled 614 patients (training dataset: n = 390, internal validation dataset: n = 98, and external validation dataset: n = 126) of T1-category NPC and NPH. Three feature selection methods were used, including analysis of variance, recursive feature elimination, and relief. The logistic regression classifier was performed to construct the radiomics signatures of T2WI-FS, CE-T1WI, and T2WI-FS + CE-T1WI to differentiate T1-category NPC from NPH. The performance of the optimal radiomics signature (T2WI-FS + CE-T1WI) was compared with those of three radiologists in the internal and external validation datasets.
Results
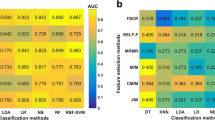
Twelve, 15, and 15 radiomics features were selected from T2WI-FS, CE-T1WI, and T2WI-FS + CE-T1WI to develop the three radiomics signatures, respectively. The area under the curve (AUC) values for radiomics signatures of T2WI-FS + CE-T1WI and CE-T1WI were significantly higher than that of T2WI-FS (AUCs = 0.940, 0.935, and 0.905, respectively) for distinguishing T1-category NPC and NPH in the training dataset (Ps all < 0.05). In the internal and external validation datasets, the radiomics signatures based on T2WI-FS + CE-T1WI and CE-T1WI outperformed T2WI-FS with no significant difference (AUCs = 0.938, 0.925, and 0.874 for internal validation dataset and 0.932, 0.918, and 0.882 for external validation dataset; Ps > 0.05). The radiomics signature of T2WI-FS + CE-T1WI significantly performed better than three radiologists in the internal and external validation datasets.
Conclusion
The MRI-based radiomics signature is meaningful in differentiating T1-category NPC from NPH and potentially helps clinicians select suitable therapy strategies.







Similar content being viewed by others
References
Guo R, Mao YP, Tang LL, Chen L, Sun Y, Ma J. The evolution of nasopharyngeal carcinoma staging. Br J Radiol. 2019;92(1102):20190244.
Petersson F. Nasopharyngeal carcinoma: a review. Semin Diagn Pathol. 2015;32(1):54–73.
King AD, Wong LYS, Law BKH, Bhatia KS, Woo JKS, Ai QY, et al. MR imaging criteria for the detection of nasopharyngeal carcinoma: discrimination of early-stage primary tumors from benign hyperplasia. AJNR Am J Neuroradiol. 2018;39(3):515–23.
Gao L, Zhou L, Huang X. Identification of novel kinase-transcription factor-mRNA-miRNA regulatory network in nasopharyngeal carcinoma by bioinformatics analysis. Int J Gen Med. 2021;14:7453–69.
Abdel Khalek Abdel Razek A, King A. MRI and CT of nasopharyngeal carcinoma. AJR Am J Roentgenol. 2012;198(1):11–8.
Wang P, **ao Z, Tang Z, Wang J. Dual-energy CT in the differentiation of stage T1 nasopharyngeal carcinoma and lymphoid hyperplasia. Eur J Radiol. 2020;124: 108824.
King AD, Woo JKS, Ai QY, Chan JSM, Lam WKJ, Tse IOL, et al. Complementary roles of MRI and endoscopic examination in the early detection of nasopharyngeal carcinoma. Ann Oncol. 2019;30(6):977–82.
Wang ML, Wei XE, Yu MM, Li WB. Value of contrast-enhanced MRI in the differentiation between nasopharyngeal lymphoid hyperplasia and T1 stage nasopharyngeal carcinoma. Radiol Med. 2017;122(10):743–51.
Zhang SX, Jia QJ, Zhang ZP, Liang CH, Chen WB, Qiu QH, et al. Intravoxel incoherent motion MRI: emerging applications for nasopharyngeal carcinoma at the primary site. Eur Radiol. 2014;24(8):1998–2004.
Ai QY, King AD, Chan JSM, Chen W, Chan KCA, Woo JKS, et al. Distinguishing early-stage nasopharyngeal carcinoma from benign hyperplasia using intravoxel incoherent motion diffusion-weighted MRI. Eur Radiol. 2019;29(10):5627–34.
Yu JY, Zhang D, Huang XL, Ma J, Yang C, Li XJ, et al. Quantitative analysis of DCE-MRI and RESOLVE-DWI for differentiating nasopharyngeal carcinoma from nasopharyngeal lymphoid hyperplasia. J Med Syst. 2020;44(4):75.
Meng T, He H, Liu H, Lv X, Huang C, Zhong L, et al. Investigation of the feasibility of synthetic MRI in the differential diagnosis of non-keratinising nasopharyngeal carcinoma and benign hyperplasia using different contoured methods for delineation of the region of interest. Clin Radiol. 2021;76(3):238 e9-338.
**ao B, Wang P, Zhao Y, Liu Y, Ye Z. Using arterial spin labeling blood flow and its histogram analysis to distinguish early-stage nasopharyngeal carcinoma from lymphoid hyperplasia. Medicine (Baltimore). 2021;100(8): e24955.
Ke L, Deng Y, **a W, Qiang M, Chen X, Liu K, et al. Development of a self-constrained 3D DenseNet model in automatic detection and segmentation of nasopharyngeal carcinoma using magnetic resonance images. Oral Oncol. 2020;110: 104862.
Deng Y, Li C, Lv X, **a W, Shen L, **g B, et al. The contrast-enhanced MRI can be substituted by unenhanced MRI in identifying and automatically segmenting primary nasopharyngeal carcinoma with the aid of deep learning models: an exploratory study in large-scale population of endemic area. Comput Methods Programs Biomed. 2022;217: 106702.
Wong LM, King AD, Ai QYH, Lam WKJ, Poon DMC, Ma BBY, et al. Convolutional neural network for discriminating nasopharyngeal carcinoma and benign hyperplasia on MRI. Eur Radiol. 2021;31(6):3856–63.
Wong LM, Ai QYH, Zhang R, Mo F, King AD. Radiomics for discrimination between early-stage nasopharyngeal carcinoma and benign hyperplasia with stable feature selection on MRI. Cancers (Basel). 2022;14(14):3433.
Chan KCA, Woo JKS, King A, Zee BCY, Lam WKJ, Chan SL, et al. Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med. 2017;377(6):513–22.
Pan JJ, Ng WT, Zong JF, Chan LLK, O’Sullivan B, Lin SJ, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity‐modulated radiotherapy. Cancer. 2016;122(4):546–58. https://doi.org/10.1002/cncr.29795.
Wang X, Dai S, Wang Q, Chai X, **an J. Investigation of MRI-based radiomics model in differentiation between sinonasal primary lymphomas and squamous cell carcinomas. Jpn J Radiol. 2021;39(8):755–62.
Bao D, Liu Z, Geng Y, Li L, Xu H, Zhang Y, et al. Baseline MRI-based radiomics model assisted predicting disease progression in nasopharyngeal carcinoma patients with complete response after treatment. Cancer Imaging. 2022;22(1):10.
Song Y, Zhang J, Zhang YD, Hou Y, Yan X, Wang Y, et al. FeAture Explorer (FAE): a tool for develo** and comparing radiomics models. PLoS ONE. 2020;15(8): e0237587.
van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77(21):e104–7.
Yang P, Xu L, Cao Z, Wan Y, Xue Y, Jiang Y, et al. Extracting and selecting robust radiomic features from PET/MR Images in nasopharyngeal carcinoma. Mol Imaging Biol. 2020;22(6):1581–91.
Chen C, Qin Y, Chen H, Cheng J, He B, Wan Y, et al. Machine learning to differentiate small round cell malignant tumors and non-small round cell malignant tumors of the nasal and paranasal sinuses using apparent diffusion coefficient values. Eur Radiol. 2022;32(6):3819–29.
Yardimci AH, Kocak B, Sel I, Bulut H, Bektas CT, Cin M, et al. Radiomics of locally advanced rectal cancer: machine learning-based prediction of response to neoadjuvant chemoradiotherapy using pre-treatment sagittal T2-weighted MRI. Jpn J Radiol. 2023;41(1):71–82.
Miranda D, Olivares R, Munoz R, Minonzio JG. Improvement of patient classification using feature selection applied to bidirectional axial transmission. IEEE Trans Ultrason Ferroelectr Freq Control. 2022;69(9):2663–71.
Lin X, Yang F, Zhou L, Yin P, Kong H, **ng W, et al. A support vector machine-recursive feature elimination feature selection method based on artificial contrast variables and mutual information. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;910:149–55.
Urbanowicz RJ, Meeker M, La Cava W, Olson RS, Moore JH. Relief-based feature selection: introduction and review. J Biomed Inform. 2018;85:189–203.
Zheng Y, Zhou D, Liu H, Wen M. CT-based radiomics analysis of different machine learning models for differentiating benign and malignant parotid tumors. Eur Radiol. 2022;32(10):6953–64.
Guang-Wu H, Sunagawa M, Jie-En L, Shimada S, Gang Z, Tokeshi Y, et al. The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope. 2000;110(12):2066–9.
Bangiyev L, Raz E, Block TK, Hagiwara M, Wu X, Yu E, et al. Evaluation of the orbit using contrast-enhanced radial 3D fat-suppressed T1 weighted gradient echo (Radial-VIBE) sequence. Br J Radiol. 2015;88(1054):20140863.
King AD, Lam WW, Leung SF, Chan YL, Metreweli C. Comparison of T2 weighted fat suppressed turbo spin echo and contrast enhanced T1 weighted spin echo MRI in nasopharyngeal carcinoma. Br J Radiol. 1997;70(840):1208–14.
Duron L, Heraud A, Charbonneau F, Zmuda M, Savatovsky J, Fournier L, et al. A magnetic resonance imaging radiomics signature to distinguish benign from malignant orbital lesions. Invest Radiol. 2021;56(3):173–80.
Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2016;278(2):563–77.
Dehghani Firouzabadi F, Gopal N, Homayounieh F, Anari PY, Li X, Ball MW, et al. CT radiomics for differentiating oncocytoma from renal cell carcinomas: systematic review and meta-analysis. Clin Imaging. 2023;94:9–17.
Acknowledgements
This work was supported by the “Excellent doctor-Excellent Clinical Researcher” Project of Eye and ENT Hospital, Fudan University (grant number SYA202007) and “Keqing-Deji” science and technology innovation project of Fudan University (grant number SCH6222206A/022).
Funding
This work was supported by the “Excellent doctor-Excellent Clinical Researcher” Project of Eye and ENT Hospital, Fudan University (grant number SYA202007) and “Keqing-Deji” science and technology innovation project of Fudan University (grant number SCH6222206A/022).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection and analysis were performed by JC, WS, YW, YZ, YW, SY, YY, LC, and ZW. The first draft of the manuscript was written by JC and all authors commented on the previous versions of the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Ethical approval
This retrospective study was approved by the ethics committee of participating hospitals, which waived the need for informed consent from individual patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Cheng, J., Su, W., Wang, Y. et al. Magnetic resonance imaging based on radiomics for differentiating T1-category nasopharyngeal carcinoma from nasopharyngeal lymphoid hyperplasia: a multicenter study. Jpn J Radiol 42, 709–719 (2024). https://doi.org/10.1007/s11604-024-01544-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-024-01544-0