Abstract
Objective
Intrauterine growth restriction followed by postnatal catch-up growth (CG-IUGR) increases the risk of insulin resistance-related diseases. Low-density lipoprotein receptor-related protein 6 (LRP6) plays a substantial role in glucose metabolism. However, whether LRP6 is involved in the insulin resistance of CG-IUGR is unclear. This study aimed to explore the role of LRP6 in insulin signaling in response to CG-IUGR.
Methods
The CG-IUGR rat model was established via a maternal gestational nutritional restriction followed by postnatal litter size reduction. The mRNA and protein expression of the components in the insulin pathway, LRP6/β-catenin and mammalian target of rapamycin (mTOR)/S6 kinase (S6K) signaling, was determined. Liver tissues were immunostained for the expression of LRP6 and β-catenin. LRP6 was overexpressed or silenced in primary hepatocytes to explore its role in insulin signaling.
Results
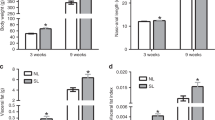
Compared with the control rats, CG-IUGR rats showed higher homeostasis model assessment for insulin resistance (HOMA-IR) index and fasting insulin level, decreased insulin signaling, reduced mTOR/S6K/ insulin receptor substrate-1 (IRS-1) serine307 activity, and decreased LRP6/β-catenin in the liver tissue. The knockdown of LRP6 in hepatocytes from appropriate-for-gestational-age (AGA) rats led to reductions in insulin receptor (IR) signaling and mTOR/S6K/IRS-1 serine307 activity. In contrast, LRP6 overexpression in hepatocytes of CG-IUGR rats resulted in elevated IR signaling and mTOR/S6K/IRS-1 serine307 activity.
Conclusion
LRP6 regulated the insulin signaling in the CG-IUGR rats via two distinct pathways, IR and mTOR-S6K signaling. LRP6 may be a potential therapeutic target for insulin resistance in CG-IUGR individuals.
Similar content being viewed by others
References
Sharma D, Shastri S, Farahbakhsh N, et al. Intrauterine growth restriction — part 1. J Matern Fetal Neonatal Med, 2016,29(24):3977–3987
**e X, Lin T, Zhang M, et al. IUGR with infantile overnutrition programs an insulin-resistant phenotype through DNA methylation of peroxisome proliferator-activated receptor-γ coactivator-1α in rats. Pediatr Res, 2015,77(5):625–632
Casey PH, Whiteside-mansell L, Barrett K, et al. Impact of prenatal and/or postnatal growth problems in low birth weight preterm infants on school-age outcomes: an 8-year longitudinal evaluation. Pediatrics, 2006,118(3):1078–1086
Ong KK. Catch-up growth in small for gestational age babies: good or bad? Curr Opin Endocrinol Diabetes Obes, 2007,14(1):30–34
Hermann GM, Miller RL, Erkonen GE, et al. Neonatal catch up growth increases diabetes susceptibility but improves behavioral and cardiovascular outcomes of low birth weight male mice. Pediatr Res, 2009,66(1):53–58
Fernandez-twinn DS, Ozanne SE. Early life nutrition and metabolic programming. Ann N Y Acad Sci, 2010,1212:78–96
Cianfarani S, Agostoni C, Bedogni G, et al. Effect of intrauterine growth retardation on liver and long-term metabolic risk. Int J Obes, 2012,36(10):1270–1277
Berends LM, Fernandez-twinn DS, Martin-gronert MS, et al. Catch-up growth following intra-uterine growth-restriction programmes an insulin-resistant phenotype in adipose tissue. Int J Obes, 2013,37(8):1051–1057
Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes, 2001,50(10):2279–2286
Berends LM, Dearden L, Tung YCL, et al. Programming of central and peripheral insulin resistance by low birthweight and postnatal catch-up growth in male mice. Diabetologia, 2018,61(10):2225–2234
Ong KK, Ahmed ML, Emmett PM, et al. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ, 2000,320(7240):967–971
Duan C, Liu M, Xu H, et al. Decreased expression of GLUT4 in male CG-IUGR rats may play a vital role in their increased susceptibility to diabetes mellitus in adulthood. Acta biochimica et biophysica Sinica, 2016,48(10):872–882
Hofman PL, Regan F, Harris M, et al. The metabolic consequences of prematurity. Growth Horm IGF Res, 2004,14(Suppl A):S136–139
Mericq V. Low birth weight and endocrine dysfunction in postnatal life. Pediatr Endocrinol Rev, 2006,4(1):3–14
Longo S, Bollani L, Decembrino L, et al. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med, 2013,26(3):222–225
Abou Ziki MD, Mani A. The interplay of canonical and noncanonical Wnt signaling in metabolic syndrome. Nutr Res, 2019,70:18–25
Liu W, Singh R, Choi CS, et al. Low density lipoprotein (LDL) receptor-related protein 6 (LRP6) regulates body fat and glucose homeostasis by modulating nutrient sensing pathways and mitochondrial energy expenditure. J Biol Chem, 2012,287(10):7213–7223
Au DT, Migliorini M, Strickland DK, et al. Macrophage LRP1 Promotes Diet-Induced Hepatic Inflammation and Metabolic Dysfunction by Modulating Wnt Signaling. Mediators Inflamm, 2018,2018:790–2841
Wang ZM, Luo JQ, Xu LY, et al. Harnessing low-density lipoprotein receptor protein 6 (LRP6) genetic variation and Wnt signaling for innovative diagnostics in complex diseases. Pharmacogenomics J, 2018,18(3):351–358
Singh R, Smith E, Fathzadeh M, et al. Rare nonconservative LRP6 mutations are associated with metabolic syndrome. Hum Mutat, 2013,34(9):1221–1225
Singh R, De Aguiar RB, Naik S, et al. LRP6 enhances glucose metabolism by promoting TCF7L2-dependent insulin receptor expression and IGF receptor stabilization in humans. Cell Metab, 2013,17(2):197–209
Mao Z, Zhang W. Role of mTOR in Glucose and Lipid Metabolism. Int J Mol Sci, 2018,19(7):2043
Yoon MS. The Role of Mammalian Target of Rapamycin (mTOR) in Insulin Signaling. Nutrients, 2017,9(11):1176
Um SH, Frigerio F, Watanabe M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature, 2004,431(7005):200–205
Gual P, Le Marchand-brustel Y, Tanti J F. Positive and negative regulation of insulin signaling through IRS-1 phosphorylation. Biochimie, 2005,87(1):99–109
Rui L, Aguirre V, Kim JK, et al. Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest, 2001,107(2):181–189
Kang S. Low-density lipoprotein receptor-related protein 6-mediated signaling pathways and associated cardiovascular diseases: diagnostic and therapeutic opportunities. Hum Genet, 2020,139(4):447–459
Li L, Xue J, Wan J, et al. LRP6 Knockdown Ameliorates Insulin Resistance via Modulation of Autophagy by Regulating GSK3β Signaling in Human LO2 Hepatocytes. Front Endocrinol (Lausanne), 2019,10:73
Santoleri D, Titchenell PM. Resolving the Paradox of Hepatic Insulin Resistance. Cell Mol Gastroenterol Hepatol, 2019,7(2):447–456
Ferris HA, Kahn CR. Unraveling the Paradox of Selective Insulin Resistance in the Liver: the Brain-Liver Connection. Diabetes, 2016,65(6):1481–1483
Liao L, Zheng R, Wang C, et al. The influence of down-regulation of suppressor of cellular signaling proteins by RNAi on glucose transport of intrauterine growth retardation rats. Pediatr Res, 2011, 69(6):497–503
Ye J, Zheng R, Wang Q, et al. Downregulating SOCS3 with siRNA ameliorates insulin signaling and glucose metabolism in hepatocytes of IUGR rats with catch-up growth. Pediatr Res, 2012,72(6):550–559
Zheng RD, Liao LH, Ye J, et al. Effects of SOCS 1/3 gene silencing on the expression of C/EBPα and PPARγ during differentiation and maturation of rat preadipocytes. Pediatr Res, 2013,73(3):263–267
Haffner SM, Miettinen H, Stern MP. The homeostasis model in the San Antonio Heart Study. Diabetes Care, 1997,20(7):1087–1092
Shen L, Hillebrand A, Wang DQ, et al. Isolation and primary culture of rat hepatic cells. J Vis Exp, 2012,(64):3917
Xu Z, He X, Shi X, et al. Analysis of differentially expressed genes among human hair follicle-derived iPSCs, induced hepatocyte-like cells, and primary hepatocytes. Stem Cell Res Ther, 2018,9(1):211
Horai S, Yanagi K, Kaname T, et al. Establishment of a primary hepatocyte culture from the small Indian mongoose (Herpestes auropunctatus) and distribution of mercury in liver tissue. Ecotoxicology, 2014,23(9):1681–1689
Bae EJ, Yang YM, Kim JW, et al. Identification of a novel class of dithiolethiones that prevent hepatic insulin resistance via the adenosine monophosphate-activated protein kinase-p70 ribosomal S6 kinase-1 pathway. Hepatology, 2007,46(3):730–739
He B, Wen Y, Hu S, et al. Prenatal caffeine exposure induces liver developmental dysfunction in offspring rats. J Endocrinol, 2019,242(3):211–226
Youngren JF. Regulation of insulin receptor function. Cell Mol Life Sci, 2007,64(7–8):873–891
Draznin B. Molecular mechanisms of insulin resistance: serine phosphorylation of insulin receptor substrate-1 and increased expression of p85alpha: the two sides of a coin. Diabetes, 2006,55(8):2392–2397
Smadja-lamère N, Shum M, Déléris P, et al. Insulin activates RSK (p90 ribosomal S6 kinase) to trigger a new negative feedback loop that regulates insulin signaling for glucose metabolism. J Biol Chem, 2013,288(43):31165–31176
Chen Y, Huang L, Qi X, et al. Insulin Receptor Trafficking: Consequences for Insulin Sensitivity and Diabetes. Int J Mol Sci, 2019,20(20):5007
Ferenc K, Pietrzak P, Wierzbicka M, et al. Alterations in the liver of intrauterine growth retarded piglets may predispose to development of insulin resistance and obesity in later life. J Physiol Pharmacol, 2018,69(2)
Swe MT, Pongchaidecha A, Chatsudthipong V, et al. Molecular signaling mechanisms of renal gluconeogenesis in nondiabetic and diabetic conditions. J Cell Physiol, 2019,234(6):8134–8151
Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab, 2003,285(4):E685–E692
Mericq V, Martinez-aguayo A, Uauy R, et al. Long-term metabolic risk among children born premature or small for gestational age. Nat Rev Endocrinol, 2017,13(1):50–62
Michael MD, Kulkarni RN, Postic C, et al. Loss of insulin signaling in hepatocytes leads to severe insulin resistance and progressive hepatic dysfunction. Mol Cell, 2000,6(1):87–97
Zhang W, Shen XY, Zhang WW, et al. Di-(2-ethylhexyl) phthalate could disrupt the insulin signaling pathway in liver of SD rats and L02 cells via PPARγ. Toxicol Appl Pharmacol, 2017,316:17–26
Cheng PW, Chen YY, Cheng WH, et al. Wnt Signaling Regulates Blood Pressure by Downregulating a GSK-3β-Mediated Pathway to Enhance Insulin Signaling in the Central Nervous System. Diabetes, 2015,64(10):3413–3424
Li R, Ou J, Li L, et al. The Wnt Signaling Pathway Effector TCF7L2 Mediates Olanzapine-Induced Weight Gain and Insulin Resistance. Front Pharmacol, 2018,9:379
Karczewska-kupczewska M, Stefanowicz M, Matulewicz N, et al. Wnt Signaling Genes in Adipose Tissue and Skeletal Muscle of Humans With Different Degrees of Insulin Sensitivity. J Clin Endocrinol Metabol, 2016,101(8):3079–3087
Palsgaard J, Emanuelli B, Winnay JN, et al. Cross-talk between insulin and Wnt signaling in preadipocytes: role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5). J Biol Chem, 2012,287(15):12016–12026
Yoon JC, Ng A, Kim BH, et al. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev, 2010,24(14):1507–1518
Mani A, Radhakrishnan J, Wang H, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science, 2007,315(5816):1278–1282
Bronsveld HK, De Bruin ML, Wesseling J, et al. The association of diabetes mellitus and insulin treatment with expression of insulin-related proteins in breast tumors. BMC Cancer, 2018,18(1):224
Qiao S, Mao G, Li H, et al. DPP-4 Inhibitor Sitagliptin Improves Cardiac Function and Glucose Homeostasis and Ameliorates β-Cell Dysfunction Together with Reducing S6K1 Activation and IRS-1 and IRS-2 Degradation in Obesity Female Mice. J Diabetes Res, 2018,2018:3641516
Guiducci L, Iervasi G, Quinones-galvan A. On the paradox insulin resistance/insulin hypersensitivity and obesity: two tales of the same history. Expert Rev Cardiovasc Ther, 2014,12(6):637–642
Ehrenkranz RA, Dusick AM, Vohr BR, et al. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics, 2006,117(4):1253–1261
Cunha AC, Pereira RO, Pereira MJ, et al. Long-term effects of overfeeding during lactation on insulin secretion—the role of GLUT-2. J Nutr Biochem, 2009,20(6):435–442
Conceicao EP, Trevenzoli IH, Oliveira E, et al. Higher white adipocyte area and lower leptin production in adult rats overfed during lactation. Horm Metab Res, 2011,43(7):513–516
Faienza M F, Brunetti G, Ventura A, et al. Nonalcoholic fatty liver disease in prepubertal children born small for gestational age: influence of rapid weight catch-up growth. Horm Research in Paediatr, 2013,79(2):103–109
Author information
Authors and Affiliations
Corresponding authors
Additional information
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
This work was supported by the National Natural Science Foundation of China (No. 82001651 and No. 81660268).
Rights and permissions
About this article
Cite this article
**e, Xm., Cao, Ql., Sun, Yj. et al. LRP6 Bidirectionally Regulates Insulin Sensitivity through Insulin Receptor and S6K Signaling in Rats with CG-IUGR. CURR MED SCI 43, 274–283 (2023). https://doi.org/10.1007/s11596-022-2683-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11596-022-2683-4