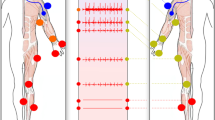
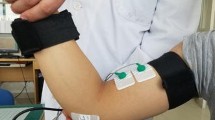
Abstract
Surface electromyography (sEMG) is a method involving physiological signals with a complex behavior. The aim is to analyze the sEMG signals by nonlinear techniques for investigating the possible neuroprotective effect of citicoline for early period of administration in rat sciatic nerve crush injury. Thirty-two Wistar rats were randomized into four groups: the sham-operated group with the intact sciatic nerve and the sciatic nerve crush groups, which received crush on the left sciatic nerve and administrated i.p. citicoline (50 and 250 mg/kg/day, 7 day) or saline (control group). Function assessment analysis was performed and sEMG signals were recorded and analyzed with nonlinear methods. Citicoline administration improved functional recovery in comparison with control group. Largest Lyapunov exponent and correlation dimension parameters were decreased due to the crush injury and increased related with the healing of sciatic nerve. Results of nonlinear analysis of sEMG are in line with the results of functional recovery and electrophysiological assessments. These results suggest that administration of citicoline protects the sciatic nerve from the crush injury which may be attributed to its antioxidative properties. Nonlinear analysis of sEMG is a promising supporting method for determining the nerve regeneration process during the treatment of peripheral nerve injuries.
Graphical abstract




Similar content being viewed by others
References
Padmanabhan P, Puthusserypady S (2009) Nonlinear analysis of EMG signals - a chaotic approach. Conf Proc IEEE Eng Med Biol Soc 2006:608–611. https://doi.org/10.1109/IEMBS.2004.1403231
Aziz S, Khan MU, Aamir F et al (2019) Electromyography (EMG) data-driven load classification using empirical mode decomposition and feature analysis. 2019 Int Conf Front Inf Technol (FIT) 272–2725. https://doi.org/10.1109/FIT47737.2019.00058.
Hudgins B, Parker P, Scott R (1993) A new strategy for multifunction myoelectric control. IEEE Trans Biomed Eng 40:82–94
Farina D, Merletti R, Nazzaro M et al (2001) Effect of joint angle on EMG variables in leg and thigh muscles. IEEE Eng Med Biol Mag 20(6):62–71. https://doi.org/10.1109/51.982277
Englehart K, Hudgin B, Parker P (2001) A wavelet-based continuous classification scheme for multifunction myoelectric control. IEEE Trans Biolmed Eng 48:302–311
Hong T, Zhang X, Ma H et al (2016) Fatiguing effects on the multi-scale entropy of surface electromyography in children with cerebral palsy. Entropy 18(5):177. https://doi.org/10.3390/e18050177
Talebinejad M, Chan AD, Miri A et al (2009) Fractal analysis of surface electromyography signals: a novel power spectrum-based method. J Electromyogr Kinesiol 19(5):840–50. https://doi.org/10.1016/j.jelekin.2008.05.004
Bauder AR, Ferguson TA (2012) Reproduceable mouse sciatic nerve crush and subsequent assessment of regeneration by whole mount muscle analysis. J Vis Exp 60:e3606. https://doi.org/10.3791/3606
Weiss GB (1995) Metabolism and actions of CDP-choline as an endogenously as citicoline. Life Sci 56:637–660
Özay R, Bekar A, Kocaeli H et al (2007) Citicoline improves functional recovery, promotes nerve regeneration, and reduces postoperative scaling after peripheral nerve surgery in rats. Surg Neurol 68:615–622
Aslan E, Kocaeli H, Bekar A et al (2011) CDP-choline and its endogenous metabolites, cytidine and choline, promote the nerve regeneration and improve the functional recovery of injured rat sciatic nerves. Neurol Res 33(7):766–773
Caner B, Kafa MI, Bekar A et al (2012) Intraperitoneal administration of CDP-choline or a combination of cytidine plus choline improves nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Neurol Res 34(3):238–245
Kaplan T, Kafa IM, Cansev M et al (2014) Investigation of the dose-dependency of citicoline effects on nerve regeneration and functional recovery in a rat model of sciatic nerve injury. Turk Neurosurg 24(1):54–62
Varejão AS, Melo-Pinto P, Meek MF et al (2004) Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol Res 26:186–194
Lu´ıs AL, Amado S, Geunad S et al (2007) Long-term functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurosci Methods 163:92–104
De Luca CJ, Gilmore LD, Kuznetsov M et al (2010) Filtering the surface EMG signal: movement artifact and baseline noise contamination. J Biomech 43(8):1573–1579. https://doi.org/10.1016/j.jbiomech.2010.01.027
Nodes T, Gallagher N (1982) Median filters: Some modifications and their properties. IEEE Trans Acoust 30(5):739–746. https://doi.org/10.1109/TASSP.1982.1163951
Chowdhury RH, Reaz MB, Ali MA et al (2013) Surface electromyography signal processing and classification techniques. Sensors (Basel) 13(9):12431–66. https://doi.org/10.3390/s130912431
Andrade AO, Nasuto S, Kyberd P et al (2006) EMG signal filtering based on empirical mode decomposition. Biomed Signal Process Control 1(1):44–55
Baspinar U, Senyurek VY, Dogan B et al (2015) A comparative study of denoising sEMG signals. Turk J Elec Eng Comp Sci 23:931–944
Flanders M (2002) Choosing a wavelet for single-trial EMG. J Neurosci Methods 116:165–177
Rafiee J, Rafiee MA, Prause N et al (2011) Wavelet basis functions in biomedical signal processing. Expert Syst Appl 38(5):6190–6201
Hussain MS, Reaz MBI, Mohd-Yasin F et al (2009) Electromyography signal analysis using wavelet transform and higher order statistics to determine muscle contraction. Expert Syst 26(1):35–48
Hu Z, Wang Z (2004) Detecting the motor unit action potential from surface EMG signals based on wavelet transform. IEEE Biomed Circuits Syst 6-15.https://doi.org/10.1109/BIOCAS.2004.1454166
Hu X, Wang Z, Ren X (2005) Classification of surface EMG signal using relative wavelet packet energy. Comput Methods Programs Biomed 79(3):189–195
Huang Y, Chen K, Zhang X et al (2021) Motion estimation of elbow joint from sEMG using continuous wavelet transform and back propagation neural networks. Biomed Signal Process Control 68:102657
Sobahi NM (2011) Denoising of EMG signals based on wavelet transform. Asia Trans Eng 1(5):17–23
Jiang CF, Kuo SL (2007) A comperative study of wavelet denoising of surface electromyographic signals. Annu Int Conf IEEE Eng Med Biol Soc 2007:1868–1871
He C, **ng J, Li J et al (2015) A new wavelet threshold determination method considering interscale correlation in signal denoising. Math Probl Eng 2015:280251
Priya KD, Rao GS, Rao PSVS (2016) Comparative analysis of wavelet thresholding techniques with wavelet-wiener filter on ECG signal. Procedia Comput Sci 87:178–183
Chen G, **e W, Zhao Y (2013) Wavelet-based denoising: a brief review. 2013 Fourth International Conference on Intelligent Control and Information Processing (ICICIP), 570–574
Walker JS (1999) A premier on wavelets and their scientific applications. Chapman and Hall/CRC, London
Diab A, Falou O, Hassan M et al (2015) Effect of filtering on the classification rate of nonlinear analysis methods applied to uterine EMG signals. Annu Int Conf IEEE Eng Med Biol Soc 2015:4182–4185. https://doi.org/10.1109/EMBC.2015.7319316
Takens F (1981) Detecting strange attractors in turbulence. Lect Notes Math 898:366
Fraser AM, Swinney HL (1986) Independent coordinates for strange attractors from mutual information. Phys Rev A 33(2):1134–1140
Cao L (1997) Practical method for determining the minimum embedding dimension of a scalar time series. Physica D 110(1):43–50
Abarbanel HDI (1993) The analysis of observed chaotic data in physical systems. Rev Mod Phys 65(4):1331–1392
Rosenstein MT, Collins JJ, De Luca CJ (1993) A practical method for calculating largest Lyapunov exponents from small data sets. Physica D 65:117–134
Grassberger P, Procaccia I (1983) Characterization of strange attractors. Phys Rev Lett 50(5):346–349
Pincus SM (1991) Approximate entropy as a measure of system complexity. Proc Natl Acad Sci USA 88:2297–2301
Correa PR, Catai AM, Takakura IT et al (2010) Heart rate variability and pulmonary infections after myocardial revascularization. Arq Bras Cardiol 95:448–456
Hurtado O, Lizasoain L, Moro MA (2011) Neuroprotection and recovery: recent data at the bench on citicoline. Stroke 42:S33–S35
Cetinkaya M, Cansev M, Kafa IM et al (2013) Cytidine 5’-diphosphocholine ameliorates hyperoxic lung injury in a neonatal rat model. Pediatr Res 74(1):26–33. https://doi.org/10.1038/pr.2013.68
Sobrado M, Lopez MG, Carceller F et al (2003) Combined nimodipine and citicoline reduce infarct size, attenuate apoptosis and increase Bcl-2 expression after focal cerebral ischemia. Neuroscience 118:107–113
Adibhatla RM, Hatcher JF (2002) Citicoline mechanisms and clinical efficacy in cerebral ischemia. J Neurosci Res 70:133–139
Alberghina M, Viola M, Serra I et al (1981) Effect of CDP-choline on the biosynthesis of phospholipids in brain regions during hypoxic treatment. J Neurosci Res 6:421–433
Grieb P, Rejdak R (2002) Pharmacodynamics of citicoline relevant to the treatment of glaucoma. J Neurosci Res 67:143–148
Yücel N, Cayli SR, Ateş O et al (2006) Evaluation of the neuroprotective effects of citicoline after experimental spinal cord injury: improved behavioral and neuroanatomical recovery. Neurochem Res 31:767–775
Araujo-Filho HG, Quintans-Junior LJ, Barreto AS et al (2016) Neuroprotective effect of natural products on peripheral nerve degeneration: a systematic review. Neurochem Res 41:647–658
Sun H, Liu J, Ding F et al (2006) Investigation of differentially expressed proteins in rat gastrocnemius muscle during denervation-reinnervation. J Muscle Res Cell Motil 27:241–250
Wang Y, Wang H, Mi D et al (2015) Periodical assessment of electrophysiological recovery following sciatic nerve crush via surface stimulation in rats. Neurol Sci 36:449–456
Leal-Cardoso JH, Matos-Brito BG, Lopes-Junior JEG et al (2004) Effects of estragole on the compound action potential of the rat sciatic nerve. Braz J Med Biol Res 37:1193–1198
Lin H, Wang H, Chen D et al (2007) A dose-effect relationship of ginkgo biloba extract to nerve regeneration in a rat model. Microsurgery 27:673–677
Acharya UR, Faust O, Sreec V et al (2014) Linear and nonlinear analysis of normal and CAD-affected heart rate signals. Comput Methods Programs Biomed 113:55–68
Chen X, Xu Y, Tang Y et al (2013) Nonlinear dynamics of electroencephalography study in schizophrenic patients. Chin Med J 126(15):2886–2889
Caliskan SG, Bilgin MD, Polatli M (2018) Nonlinear analysis of electrodermal activity signals for healthy subjects and patients with chronic obstructive pulmonary disease. Australas Phys Eng Sci Med 41(2):487–494. https://doi.org/10.1007/s13246-018-0649-4
Funding
This work was supported by a grant from the Adnan Menderes University, Aydin, Turkey, through grant no. TPF-07016.
Author information
Authors and Affiliations
Contributions
Serife Gokce Caliskan: Conceptualization, methodology, formal analysis, investigation, data curation, writing—original draft, project administration, visualization, and writing—review and editing. Mehmet Dincer Bilgin: Conceptualization, methodology, investigation, validation, resources, supervision, funding acquisition, project administration, and writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving animals were in accordance with the ethical standards of Adnan Menderes University’s Animal Experimentation Ethics Committee (Protocol number: 2010/019).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Çalışkan, S.G., Bilgin, M.D. Nonlinear surface EMG analysis to detect the neuroprotective effect of citicoline in rat sciatic nerve crush injury. Med Biol Eng Comput 60, 2865–2875 (2022). https://doi.org/10.1007/s11517-022-02639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-022-02639-4